Abstract
Purpose
This study was aimed to identify the incidence and severity of chemotherapy-induced peripheral neuropathy (CIPN) among patients with hematologic malignancies and to examine the relationship between the quality of life (QOL) and CIPN.
Methods
A total of 66 patients with CIPN-related symptoms participated in this study. Data were collected through self-reported questionnaires consisted of the European Organization for Research and Treatment of Cancer QLQ-C30 version 3.0 and the 16-item QLQ-CIPN20. Data were analyzed with SPSS/ WIN20 for descriptive statistics using the Mann-Whitney and Kruskal-Wallis tests, and Spearman's rho.
Results
The mean lower and upper extremity scale scores were 31.95 and 23.16 respectively for the 16-item QLQ-CIPN20. The mean QLQ-C30 subcategory scores were 46.84 for global health status, 58.72 for functional scales, and 34.85 for symptom scales. The CIPN-related lower extremity scale symptoms correlated negatively with the QOL subscales. There was no correlation between CIPN-related upper extremity symptoms and health-related QOL.
Conclusion
Patients with hematologic malignancies treated with neurotoxic chemotherapeutic agents had CIPN-related symptoms in the lower extremities mainly, and their QOL functional subscale scores were relatively lower than those of other cancer patients. Interventions need to be developed for patients with hematologic malignancies to alleviate CIPN and enhance their QOL.
Go to : 
REFERENCES
1.Wickham R. Chemotherapy-induced peripheral neuropathy: a review and implications for oncology nursing practice. Clinical Journal of Oncology Nursing. 2007. 11(3):361–76.

2.Postma TJ., Aaronson NK., Heimans JJ., Muller MJ., Hildebrand JG., Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. European Journal of Cancer. 2005. 41(8):1135–39.

3.Tofthagen C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clinical Journal of Oncology Nursing. 2010. 14(3):E22–8. http://dx.doi.org/10.1188/10.CJON.E22-E28.

4.Ocean AJ., Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Supportive Care in Cancer. 2004. 12(9):619–25.

5.Kautio AL., Haanpää M., Kautiainen H., Kalso E., Saarto T. Burden of chemotherapy-induced neuropathy-a cross-sectional study. Supportive Care in Cancer. 2011. 19(12):1991–6. http://dx.doi.org/10.1007/s00520-010-1043-2.

6.Delforge M., Blade J., Dimopoulos M., Facon T., Kropff M., Ludwig H, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. The Lancet. 2010. 11(11):1086–95. http://dx.doi.org/10.1016/S1470-2045(10)70068-1.

7.Chaudhry V., Cornblath DR., Polydefkis M., Ferguson A., Borrel-lo I. Characteristics of bortezomib- and thalidomideinduced peripheral neuropathy. Journal of the Peripheral Nervous System. 2008. 13(4):275–82.

8.Richardson PG., Briemberg H., Jagannath S., Wen PY., Barlogie B., Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. Journal of Clinical Oncology. 2006. 24(19):3113–20.

9.Badros A., Goloubeva O., Dalal JS., Can I., Thompson J., Rapoport AP, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007. 110(5):1042–9.

10.Berkowitz A., Walker S. Bortezomib-induced peripheral neuropathy in patients with multiple myeloma. Clinical Journal of Oncology Nursing. 2012. 16(1):86–9. http://dx.doi.org/10.1188/12.CJON.86-89.

11.Kim JH., Choi KS., Kim TW., Hong YS. Quality of life in colorectal cancer patients with chemotherapy-induced peripheral neuropathy. Journal of Korean Oncology Nursing. 2011. 11(3):254–62. http://dx.doi.org/10.5388/jkon.2011.11.3.254.

12.Kiser DW., Greer TB., Wilmoth MC., Dmochowski J., Naumann RW. Peripheral neuropathy in patients with gynecologic cancer receiving chemotherapy: patient reports and provider assessment. Oncology Nursing Forum. 2010. 37(6):758–64. http://dx.doi.org/10.1188/10.ONF.758-764.
13.Tofthagen C., McAllister RD., McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clinical Journal of Oncology Nursing. 2011. 15(2):182–8. http://dx.doi.org/10.1188/11.CJON.182-188.

14.Visovsky C., Collins M., Abbott L., Aschenbrenner J., Hart C. Putting evidence into practice: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clinical Journal of Oncology Nursing. 2007. 11(6):901–13.

15.Barton DL., Wos EJ., Qin R., Mattar BI., Green NB., Lanier KS, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Supportive Care in Cancer. 2011. 19(6):833–41. http://dx.doi.org/10.1007/s00520-010-0911-0.

16.Clark PG., Cortese-Jimenez G., Cohen E. Effects of reiki, yoga, or meditation on the physical and psychological symptoms of chemotherapy-induced peripheral neuropathy: arandomized pilot study. Journal of Evidence-Based Complementary & Alternative Medicine. 2012. 17(3):161–71. http://dx.doi.org/10.1177/2156587212450175.
17.Donald GK., Tobin I., Stringer J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupuncture in Medicine. 2011. 29(3):230–3. http://dx.doi.org/10.1136/acupmed.2011.010025.

18.Smith EM., Pang H., Cirrincione C., Fleishman S., Paskett ED., Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Journal of the American Medical Association. 2013. 309(13):1359–67. http://dx.doi.org/10.1001/jama.2013.2813.
19.Yoon J., Jeon JH., Lee YW., Cho CK., Kwon KR., Shin JE, et al. Sweet bee venom pharmacopuncture for chemotherapy induced peripheral neuropathy. Journal of Acupuncture & Meridian Studies. 2012. 5(4):156–65. http://dx.doi.org/10.1016/j.jams.2012.05.003.
20.Cavaletti G., Cornblath DR., Merkies IS., Postma TJ., Rossi E., Fri-geni B, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Annals of Oncology. 2013. 24(2):454–62. http://dx.doi.org/10.1093/annonc/mds329.

21.Kim HY., Kang JH., Song CE., Youn HJ. Chemotherapy-induced peripheral neuropathy and quality of life in breast cancer patients. Asian Oncology Nursing. 2013. 13(4):222–30. http://dx.doi.org/10.5388/aon.2013.13.4.222.

22.Kim HY., Kang JH., Youn HJ., So HS., Song CE., Chae SY, et al. Reliability and validity of the Korean version of the European Organization for Research and Treatment of Cancer Quality of Life questionnaire to assess chemotherapy-induced peripheral neuropathy. Journal of Korean Academy of Nursing. 2014. 44(6):735–42. http://dx.doi.org/10.4040/jkan.2014.44.6.735.

23.Faul F., Erdfelder E., Buchner A., Lang AG. Statistical power analyses using GPower 3.1: tests for correlation and regression analyses. Behavior Research Methods. 2009. 41(4):1149–60.
24.Lavoie Smith EM., Barton DL., Qin R., Steen PD., Aaronson NK., Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Quality of Life Research. 2013. 22(10):2787–99. http://dx.doi.org/10.1007/s11136-013-0379-8.

25.Fayers PM., Aaronson NK., Bjordal K., Groenvold M., Curran D., Bottomley A, et al. The EORTC QLQ-C30 Scoring Manual. 3rd ed.Brussels: European Organization for Research and Treatment of Cancer;2001.
26.Yun YH., Park YS., Lee ES., Bang SM., Heo DS., Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Quality of Life Research. 2004. 13(4):863–8.

27.Wolf SL., Barton DL., Qin R., Wos EJ., Sloan JA., Liu H, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapyinduced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN instrument, N06CA. Supportive Care in Cancer. 2012. 20(3):625–32. http://dx.doi.org/10.1007/s00520-011-1141-9.
28.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatmentstrategies. European Journal of Cancer. 2008. 44:1507–15. http://dx.doi.org/10.1016/j.ejca.2008.04.018.
Go to : 
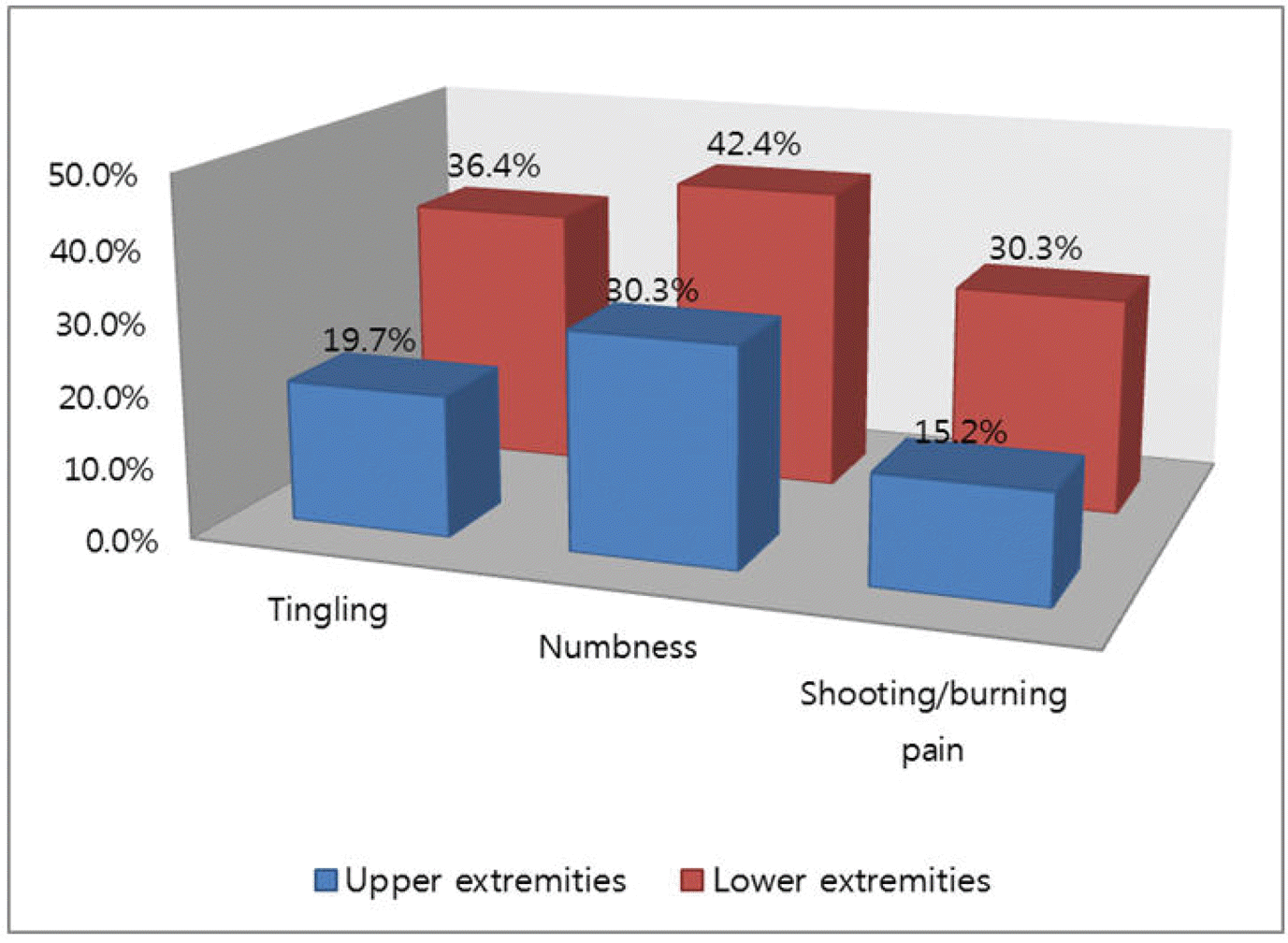 | Figure 1.Percentage of patients who reported "quite a bit" or "very much"tingling, numbness, and/or shooting/burning pain on the 16-item QLQ-CIPN20 sensory scales. |
Table 1.
Mean Rank Differences in CIPN and QOL, according to the General and Clinical Characteristics (N=66)
Table 2.
Scores from the 16-item QLQ-CIPN20 Subscales and EORTC QLQ-C30 Subscales (N=66)
Table 3.
Correlation between Health-related QOL and Peripheral Neuropathy Status (N=66)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download