Abstract
Objectives
To review the current evidence on the development of a viable surgical strategy for successful treatment of patients with osteoporotic vertebral fractures.
Summary of Literature Review
Achieving rigid and stable spinal column reconstruction in elderly patients with osteoporosis is challenging because of the poor healing capacity and weak mechanical strength of their bones.
Materials and Methods
A literature search of clinical and biomechanical studies on the issues of surgical treatment of patients with osteoporotic vertebral collapse was performed and reviewed in terms of the surgical approach, fixation, graft material, and medical considerations. Illustrative cases of the authors’ experiences were presented and reflected upon.
Results
Posterior spinal fusion and vertebral augmentation showed shorter operating times, less bleeding, and fewer complications with comparable or superior clinical results than anterior corpectomy and fusion or a posterior closing wedge vertebral shortening procedure in multiple studies. Therefore, we recommend the former as a first-line surgical plan for patients with osteoporotic vertebral collapse. However, in some patients who suffer fixed kyphosis, or spinal cord compression by a retropulsed bony fragment or bone cement, or infected vertebroplasty, an anterior approach could be considered to remove the pertinent lesion and to restore anterior spinal column. For the enhancement of the purchasing strength of the screw in the osteoporotic vertebra (e), a technique of prefilled bone cement in the instrumented vertebra(e) or injection of bone cement through a fenestrated screw is useful. Further, preoperative assessment and correction of systemic and local factors that affect bone healing is required when spinal fusion surgery is considered in elderly osteoporotic patients. The selection of the graft material should be individualized according to the property among osteoconduction, osteoinduction, and ostegenesis, or structural support that is the most important for the successful bone healing of each patient.
Conclusions
Comprehensive geriatric assessment and management of elderly patients before surgery and careful and meticulous surgical planning with respect to the surgical approach, instrumentation, and the graft material are important to achieve the best outcome of the surgical treatment of patients with osteoporotic vertebral collapse.
Go to : 
REFERENCES
1. Lill CA, Hesseln J, Schlegel U, et al. Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J Orthop Res. 2003; 21:836–42.

2. Peter Augat, Ulrich Simon, Astrid Liedert, et al. Mechan-ics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005; 16:S36–S43.

3. Ito Y, Hasegawa Y, Toda K, et al. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. 2002; 2:101–6.

4. Abdelrahman H, Siam AE, Shawky A, et al. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. 2013; 13:1809–17.

5. Kim KI, Park KH, Koo KH, et al. Comprehensive geriatric assessment can predict postoperative morbidity and mortality in elderly patients undergoing elective surgery. Arch Gerontol Geriatr. 2013; 56:507–12.

6. Kanayama M, Ishida T, Hashimoto T, et al. Role of major spine surgery using Kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech. 2010; 23:53–6.

7. Sudo H, Ito M, Kaneda K, et al. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J. 2013; 13:1726–32.

8. Moskovich R, Benson D, Zhang ZH, et al. Extracoelomic approach to the spine. J Bone Joint Surg Br. 1993; 75:886–93.

9. Nakashima H, Imagama S, Yukawa Y, et al. Comparative study of 2 surgical procedures for osteoporotic delayed vertebral collapse: anterior and posterior combined surgery versus posterior spinal fusion with vertebroplasty. Spine (Phila Pa 1976). 2015; 40:E120–6.
10. Ragel BT, Amini A, Schmidt MH. Thoracoscopic vertebral body replacement with an expandable cage after ventral spinal canal decompression. Neurosurgery. 2007; 61:317–22.

11. Uchida K, Nakajima H, Yayama T, et al. Vertebroplastyaugmented short-segment posterior fixation of osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine: comparisons with posterior surgery without vertebroplasty and anterior surgery. J Neurosurg Spine. 2010; 13:612–21.

12. Nakamae T, Fujimoto Y, Yamada K, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture with intravertebral cleft associated with delayed neurologic deficit. Eur Spine J. 2013; 22:1624–32.

13. Patil S, Rawall S, Singh D, et al. Surgical patterns in osteoporotic vertebral compression fractures. Eur Spine J. 2013; 22:883–91.

14. Sudo H, Ito M, Abumi K, et al. One-stage posterior instrumentation surgery for the treatment of osteoporotic vertebral collapse with neurological deficits. Eur Spine J. 2010; 19:907–15.

15. Kashii M, Yamazaki R, Yamashita T, et al. Surgical treatment for osteoporotic vertebral collapse with neurological deficits: retrospective comparative study of three procedures-anterior surgery versus posterior spinal shorting osteotomy versus posterior spinal fusion using vertebroplasty. Eur Spine J. 2013; 22:1633–42.

16. Okuda S, Oda T, Yamasaki R, et al. Surgical outcomes of osteoporotic vertebral collapse: a retrospective study of anterior spinal fusion and pedicle subtraction osteotomy. Global Spine J. 2012; 2:221–6.

17. Shea TM, Laun J, Gonzalez-Blohm SA, et al. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: current status. BioMed Res Int. 2014 Mar 3. [Epub ahead of print].

18. Ponnusamy KE, Iyer S, Gupta G, et al. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011; 11:54–63.

19. Kueny RA, Kolb JP, Lehmann W, et al. Influence of the screw augmentation technique and a diameter increase on pedicle screw fixation in the osteoporotic spine: pullout versus fatigue testing. Eur Spine J. 2014; 23:2196–202.

20. Hsu C-C, Chao C-K, Wang J-L, Hou S-M, Tsai Y-T, Lin J. Increase of pullout strength of spinal pedicle screws with conical core: biomechanical tests and fi-nite element analyses. Journal of Orthopaedic Research. 2005; 23(4):788–94.

21. Hirano T, Hasegawa K, Washio T, Hara T, Takahashi H. Fracture risk during pedicle screw insertion in osteoporotic spine. J Spinal Disord. 1998; 11:493–7.

22. Hirano T, Hasegawa K, Takahashi HE, et al. Structural characteristics of the pedicle and its role in screw stability. Spine. 1997; 22(21):2504–10.

23. Kwok AWL, Finkelstein JA, Woodside T, Hearn TC, Hu RW. Insertional torque and pull-out strengths of conical and cylindrical pedicle screws in cadaveric bone. Spine. 1996; 21(21):2429–34.

24. Chen L-H, Tai C-L, Lee D-M, et al. Pullout strength of pedicle screws with cement augmentation in severe osteoporosis: a comparative study between cannulated screws with cement injection and solid screws with cement pre-filling. BMC Musculoskeletal Disorders. 2011; 12:article 33.

25. Kim Y-Y, Choi W-S, Rhyu K-W. Assessment of pedicle screw pullout strength based on various screw designs and bone densities-An ex vivo biomechanical study. Spine Journal. 2012; 12(2):164–8.
26. Brasiliense LB, Lazaro BCR, Reyes M, et al. Characteristics of immediate and fatigue strength of a dual-threaded pedicle screw in cadaveric spines. Spine Journal. 2013; 13(8):947–56.

27. Christensen FB, Dalstra M, Sejling F, Overgaard S, Bü nger C. Titanium-alloy enhances bone-pedicle screw fixation: mechanical and histomorphometrical results of tita-nium-alloy versus stainless steel. European Spine Journal. 2000; 9(2):97–103.

28. Battula S, Schoenfeld AJ, Sahai V, Vrabec GA, Tank J, Njus GO. The effect of pilot hole size on the insertion torque and pullout strength of self-tapping cortical bone screws in osteoporotic bone. Journal of Trauma. 2008; 64(4):990–5.

29. Carmouche JJ, Molinari RW, Gerlinger T, Devine J, Pa-tience T. Effects of pilot hole preparation technique on pedicle screw fixation in different regions of the osteoporotic thoracic and lumbar spine. Journal of Neurosurgery. 2005; 3(5):364–70.

30. Helgeson MD, Kang DG, Lehman RA Jr., Dmitriev AE, Luhmann SJ. Tapping insertional torque allows prediction for better pedicle screw fixation and optimal screw size selection. Spine Journal. 2013; 13(8):957–65.

31. Patel PS, Shepherd DE, Hukins DW. The effect of screw insertion angle and thread type on the pullout strength of bone screws in normal and osteoporotic cancellous bone models. Med Eng Phys. 2010; 32:822–8.

32. Zindrick MR, Wiltse LL, Widell EH. A biomechanical study of intrapeduncular screw fixation in the lumbosa-cral spine. Clinical Orthopaedics and Related Research. 1986; 203:99–112.

33. Paik H, Dmitriev AE, Lehman RA Jr., et al. The biomechanical effect of pedicle screw hubbing on pullout resistance in the thoracic spine. Spine J. 2012; 12:417–24.

34. Koo KH, Yoon ST, Kim SB, et al. The Effect of Hubbing on the Pull-Out Strength of Lateral Mass Screws in the Cervical Spine: A Biomechanical Experiment. J Spinal Disord Tech. 2015; 28:E45–8.
35. Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009; 9:366–73.

36. Huang TJ, Hsu RW, Tai CL, et al. A biomechanical analysis of triangulation of anterior vertebral double-screw fixation. Clin Biomech (Bristol, Avon). 2003; 18:40–5.

37. Pare PE, Chappuis JL, Rampersaud R, et al. Biomechanical evaluation of a novel fenestrated pedicle screw augmented with bone cement in osteoporotic spines. Spine (Phila Pa1976). 2011; 36:E1210–4.
38. Ying SH, Kao HC, Chang MC, Yu WK, Wang ST, Liu CL. Fixation strength of PMMA-augmented pedicle screws after depth adjustment in a synthetic bone model of osteoporosis. Orthopedics. 2012; 3510:e1511–e1516.

39. Jensen JE, Jensen TG, Smith TK, et al. Nutrition in orthopedic surgery. J Bonr Joint Surg. 1982; 64A(9):1263.
40. Boden SD, Sumner DR. Biologic factors affecting spinal fusion and bone regeneration. Spine (Phila Pa 1976). 1995; 20(24 Suppl):102S–112S.

41. Shweikeh F, Hanna G, Bloom L, et al. Assessment of outcome following the use of recombinant human bone mor-phogenetic protein-2 for spinal fusion in the elderly population. J Neurosurg Sci. 2014. Jul 16. [Epub ahead of print].
Go to : 
 | Fig. 1.Simple radiography and magnetic resonance image (MRI) of a patient with delayed osteoporotic vertebral collapse (A). Postoperative simple radiography (B) and computed tomography (C) images of a patient who underwent anterior corpectomy/decompression and reconstruction with a femoral shaft allograft and instrumentation with triangulated dual-screw fixation with a bicortical purchase for the treatment of spinal cord compression with delayed osteoporotic vertebral collapse. |
 | Fig. 2.Preoperative and postoperative simple radiography (A, C) and MRI (B, D) of a 68-year-old female patient who had dynamic spinal cord compression due to an intravertebral instability showing a spinal cord signal change on the MRI without a significant canal compromise in the supine position. She underwent posterior decompression and fusion combined with vertebral body augmentation with polymethylmethacrylate to provide anterior spinal column support for the treatment of the osteoporotic vertebral collapse of T12. |
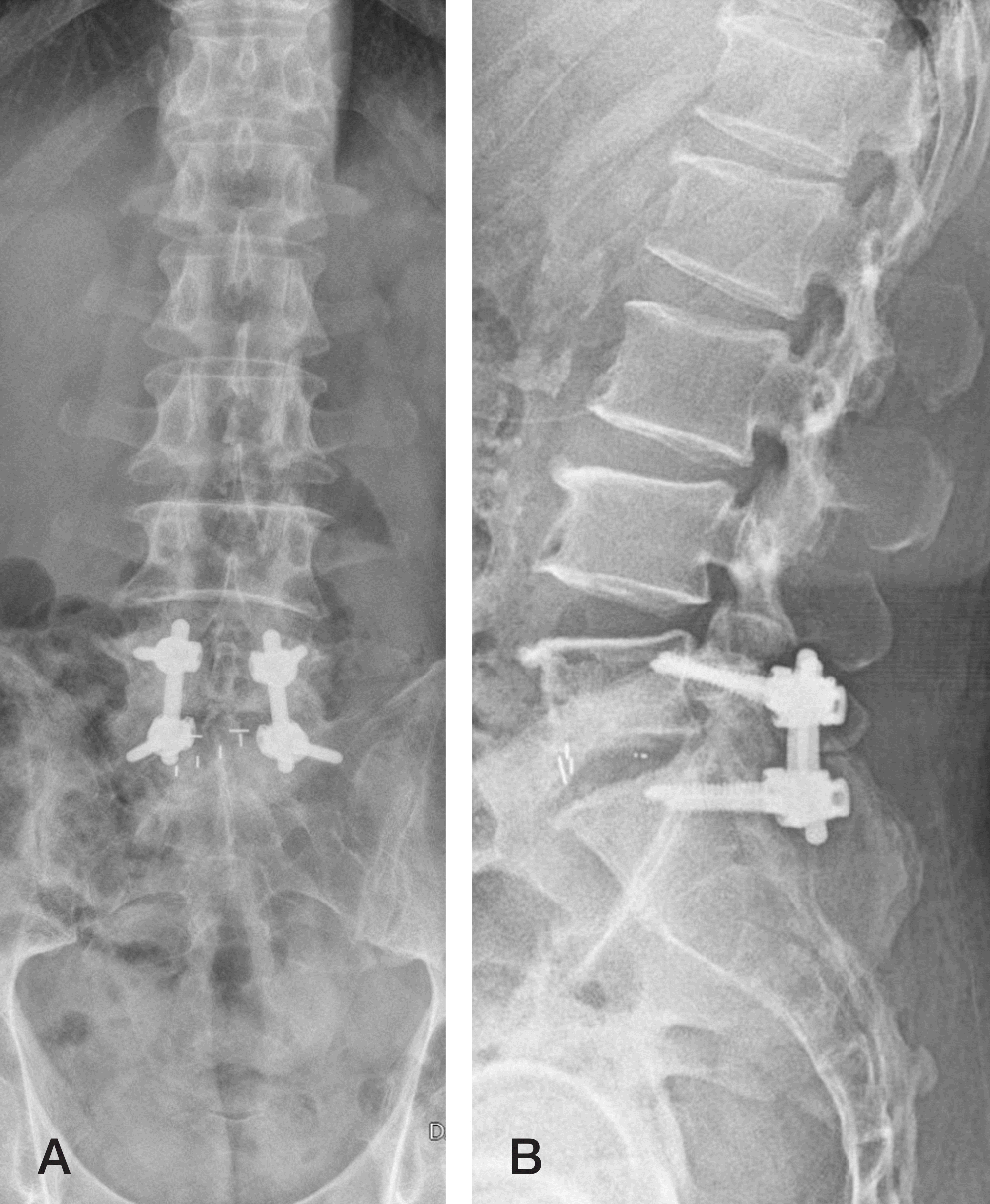 | Fig. 3.Simple radiography (A, B) showing cortical bone trajectory screw fixation for L5–S1 interbody fusion. |
Table 1.
Comparative studies according to surgical approach for treatment of patient with osteoporotic vertebral collapse.
Table 2.
Systemic factors influencing bone healing
Table 3.
Local factors influencing bone healing




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download