Abstract
Objectives
To evaluate the clinical efficacy and safety of radiofrequency (RF) ablation therapy followed by a bone cement augmentation procedure in treating and managing pain among metastatic spine tumor patients.
Summary of Literature Review
As a metastatic spine tumor is unresectable, this procedure was performed. Results showed an increase in the necrosis rate, and a decrease in local recurrence and secondary vertebral stability.
Materials and Methods
From March 2007 to April 2016, 26 patients who were treated with RF ablation with a bone cement augmentation procedure and the same number of patients treated with radiotherapy for metastatic spine lesions were included in this study. Pain relief and functional quality of life were evaluated using a visual analogue scale (VAS) and Roland Morris Questionnaire (RMQ).
Results
VAS scores preoperatively and at 1, 4, and 12 weeks follow-up were 7.45, 3.01, 3.78, and 2.97 in the procedure group, and 7.04, 6.65, 5.87, and 3.03 in the radiotherapy group. The procedure group had significantly better average outcomes than the radiotherapy group for pain relief at 4 weeks but showed no difference at 12 weeks. The RMQ score improved from 13.92 to 7.21 in the procedure group, and from 15.33 to 9.75 in the radiotherapy group. Two patients who had a metastatic tumor near the vertebral body posterior cortex showed cement leakage into the disc space, that is, intraforaminal and intracanal space; therefore, operations were performed (7.69% nerve injury).
REFERENCES
1. Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine(Phila Pa 1976). 1999; 24:1943–51.

2. Jang JH, Kim JD. Radiofrequency ablation therapy with vertebroplasty or kyphoplasty in metastatic spine tumor. J Korean Bone Joint Tumor Soc. 2007; 13:1–6.
3. Jang JH, Park YK. The comparison of the radiofrequency ablation therapy with vertebroplasty and radiotherapy in metastatic spine tumor. J Korean Orthop Assoc. 2011; 46:122–9.
4. Wright RL. Malignant tumors in the spinal extra-dural space: results of surgical treatment. Ann Surg. 1963; 157:227–31.
5. Chung CY, Lee SY, Baek GH, et al. Clinical analysis of metastatic bone tumor. J Korean Orthop Assoc. 1991; 26:1855–9.

6. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the radiation therapy oncology group. Cancer. 1982; 50:893–9.

7. Janjan NA. Radiation for bone metastases: conventional techniques and the role of systemic radiopharmaceuticals. Cancer. 1997; 80:1628–45.
8. Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine(Phila Pa 1976). 2001; 26:298–306.

9. Grö nemeyer DH, Schirp S, Gevargez A, et al. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002; 8:33–9.
10. Nakatsuka A, Yamakado K, Maeda M, et al. Radiofre-quency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004; 15:707–12.

11. Padina S, Sean D, Kieran M, et al. Comparison of the effect of two different bone-targeted radiofrequency ablation (RFA) systems alone and in combination with percutaneous vertebroplasty (PVP) on the biomechanical stability of the metastatic spine. Eur Spine J. 2015 Jul 24.

12. Huang M, Zhu H, Liu T, et al. Comparison of external radiotherapy and percutaneous vertebroplasty for spinal metastasis. Asia-Pacific Journal of Clinical Oncology. Asia Pac J Clin Oncol. 2016; 12:E201–8.
13. Erdem E, Akdol S, Amole A, et al. Radiofrequency-tar-geted vertebral augmentation for the treatment of vertebral compression fractures as a Result of Multiple Myeloma. Spine(Phila Pa 1976). 2013; 38:1275–81.

14. Pezeshki PS, Davidson SR, Akens MK, et al. Helical coil electrode radiofrequency ablation designed for application in osteolytic vertebral tumors-initial evaluation in a porcine model. Spine J. 2015; 15:1832–40.

15. Padina S, Jason Woo, Margarete K, et al. Evaluation of a bipolar-cooled radiofrequency device for ablation of bone metastasis: preclinical assessment in porcine vertebrae. Spine J. 2014; 14:70.
Fig. 1.
Postoperative anteroposterior (A) and lateral (B) radiographs of a 66-year-old female treated with radiofrequency ablation with vertebroplasty for uterine cancer spine bone metastasis at T6, L5, and S1. Her last follow-up anteroposterior (C) and lateral (D) radiographs 8 years later show some bone subsidence.

Fig. 2.
Preoperative (A, B) and postoperative (C, D) anteroposterior (A) and lateral (B, C, D) radiographs of a 34-year-old male treated with radiofre-quency ablation with vertebroplasty for mesenchymal chondrosarcoma spine bone metastasis at L2, L3, S2, and S3 with an osteoclastic lesion. Leakage of bone cement into the intracanal and intraforaminal space (C) irritated the L2 and L3 nerves and induced back pain, so an L2/L3 partial laminectomy for decompression (D) was performed 28 days later.
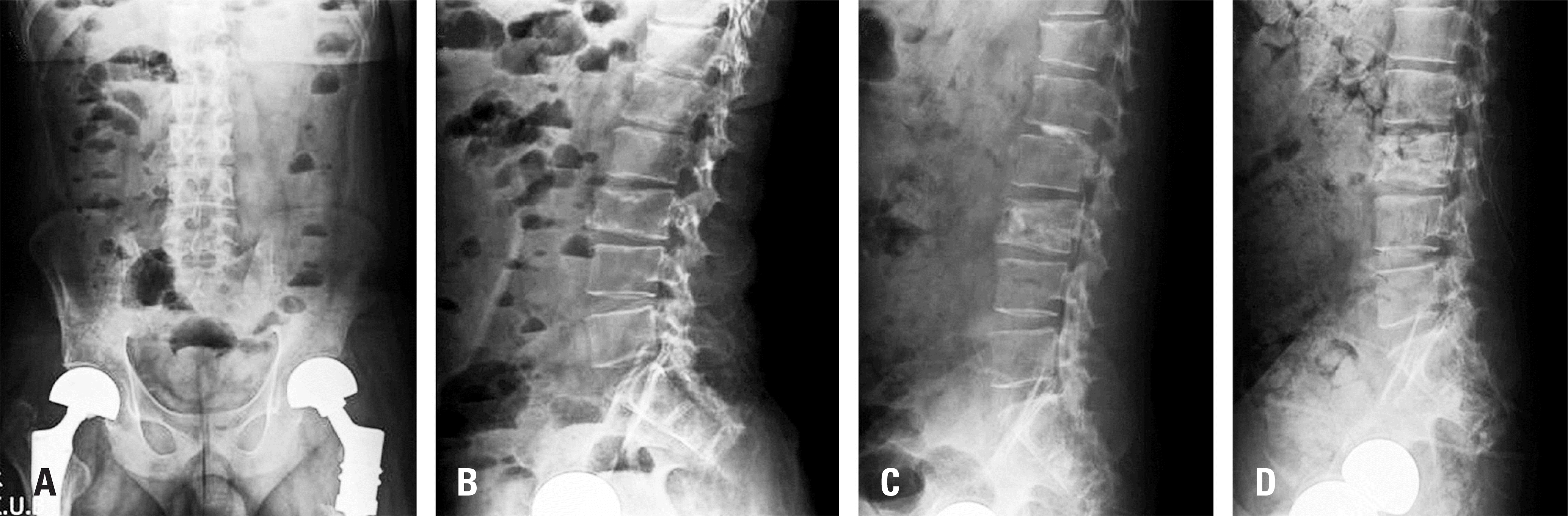
Fig. 3.
Preoperative (A, B) and postoperative (C, D) anteroposterior (A) and lateral (B, C, D) radiographs of a 72-year-old female treated by radiofre-quency ablation with vertebroplasty for urothelial cell carcinoma spine bone metastasis at T12, L2, L3, and L5 with an osteoblastic lesion. Leakage of bone cement into the intracanal and intraforaminal space (C, D) was noted, so T12/L1 partial laminectomy for decompression was performed just after the procedure.

Table 1.
Demographic chart of the RF∗ ablation with cement augmentation group
| No | Age/Sex | Tumor origin | Cell type | Spine Meta Location | Frequency of operation | Expire |
|---|---|---|---|---|---|---|
| 1 | 57/M | Breast | Ductal ca. | L1, L2, L3, T7 | 2 | 0 |
| 2 | 55/M | Breast | Ductal ca. | T9, T10, T11 | 1 | 0 |
| 3 | 28/M | OSA|| | Osteoblastic | L3, L5 | 1 | 0 |
| 4 | 57/M | Esophageal | Small cell ca. | T3, T10, T11, S1 | 1 | 0 |
| 5 | 71/M | Lung | NSCLC† (adeno ca.) | T9, L3 | 2 | 0 |
| 6 | 54/M | Rectal | Adeno ca. | L4, L5 | 1 | |
| 7 | 57/M | Lung | NSCLC (adeno ca.) | L5 | 1 | 0 |
| 8 | 47/M | Liver | HCC | T5 | 1 | 0 |
| 9 | 67/M | Lung | NSCLC (adeno ca.) | L1, L2, L4 | 1 | 0 |
| 10 | 66/F | Uterine | Squamous cell ca. | T12, L5, S1 | 1 | |
| 11 | 78/M | Prostate | L3 | 1 | ||
| 12 | 77/F | Thyroid | Papillary ca. | T11, L1 | 1 | |
| 13 | 55/M | Liver | HCC‡ | T12 | 1 | |
| 14 | 58/F | Lung | SCLC§ (atypical) | C3, T1, T4, T10, T11, L2, L3, L4, L5 | 1 | |
| 15 | 57/M | Pancreatic | Adeno ca. | C5, T10, L4, L5 | 1 | |
| 16 | 62/M | Rectal | Adeno ca. | L2 | 1 | |
| 17 | 52/F | Stomach | Adeno ca. | T7 | 1 | 0 |
| 18 | 68/M | Renal | Adeno ca. | L1, L2, S1 | 1 | 0 |
| 19 | 52/M | Rectal | Adeno ca. | L4 | 1 | |
| 20 | 67/M | Rectal | Adeno ca. | T6, L3 | 1 | |
| 21 | 57/M | Rectal | Adeno ca. | T10, L1 | 1 | |
| 22 | 63/M | Lung | NSCLC (adeno ca.) | L3 | 1 | 0 |
| 23 | 34/M | Chondrosarcoma | Mesenchymal | L2, L3, S2, S3 | 1 | 0 |
| 24 | 57/F | Thyroid | Papillary ca. | T3, T10, L3 | 1 | |
| 25 | 73/M | Renal | Adeno ca. | S2 | 1 | 0 |
| 26 | 72/F | Urothelial cell carcino | oma Squamous ca. | T12, L2, L3, L5 | 1 |
Table 2.
Demographic chart of the radiotherapy group
| No | Age/Sex | Tumor origin | Cell type | Spine Meta Location | Frequency of RTx. | Expire |
|---|---|---|---|---|---|---|
| 1 | 60/M | Liver | HCC† | T10, T11, T12 | 10 | 0 |
| 2 | 65/F | Myeloma | Multiple myeloma | C5, C6, C7 | 10 | |
| 3 | 57/M | Lung | NSCLC∗ (SQLC) | T9, L3 | 20 | 0 |
| 4 | 53/F | Breast | Ductal ca. | T9, L1, L5 | 30 | |
| 5 | 62/F | Breast | Ductal ca. | L2, L3, L4 | 10 | |
| 6 | 71/F | Rectum | Adeno ca. | L2 | 10 | |
| 7 | 52/F | Breast | Ductal ca. | L2, T1 | 20 | |
| 8 | 71/F | Lung | NSCLC (adeno ca.) | L4, S1 | 10 | 0 |
| 9 | 59/M | Lung | NSCLC (adeno ca.) | L3, L4, L5 | 30 | |
| 10 | 83/F | Lung | NSCLC | T12, L1, L2 | 10 | |
| 11 | 52/F | Lung | NSCLC (adeno ca.) | L3, L5 | 20 | 0 |
| 12 | 36/M | Pancrease | Adeno ca. | L2 | 10 | 0 |
| 13 | 57/M | Lung | NSCLC (adeno ca.) | T3, T6, T10, T12, L3, L4, L5 | 20 | |
| 14 | 47/M | Lung | NSCLC (adeno ca.) | T11, T12, L1, L4 | 30 | |
| 15 | 57/M | Stomach | Adeno ca. | T9 | 10 | |
| 16 | 60/M | Renal | RCC (papillary type) | T1, T2, T3, T4, T7, T8, T9, T12, L2, L3 | 10 | 0 |
| 17 | 55/M | Lung | SCLC‡ | T9, T11, L1 | 10 | 0 |
| 18 | 33/F | OSA | Fibroblastic | L1, L2 | 10 | |
| 19 | 47/M | Rectal | Adeno ca. | L5 | 10 | 0 |
| 20 | 25/M | Renal | RCC§ (papillary type) | T12, L4 | 20 | 0 |
| 21 | 74/M | Thyroid | Papillary ca. | T4, T5, T6 | 10 | |
| 22 | 53/F | Breast | Ductal ca. | L3, S3 | 30 | 0 |
| 23 | 59/M | liver | Ductal ca. | L2, L3, S2, S3 | 20 | 0 |
| 24 | 62/F | Breast | Ductal ca. | T4, T11, T12 | 10 | |
| 25 | 73/F | Breast | Ductal ca. | S1, S2, S3 | 10 | 0 |
| 26 | 64/M | Lung | NSCLC (adeno ca.) | T12, L3, L5 | 20 |
Table 3.
Pain Relief compared with both groups by VAS score
| VAS | |||||||
|---|---|---|---|---|---|---|---|
| Pre-OP | POD 1 week | POD 4 weeks | POD 12 weeks | p-value§ | p for interaction§ | ||
| Group A† | 7.45 | 3.01 | 3.78 | 2.97 | <0.001 | 0.004 | |
| Group B‡ | 7.04 | 6.65 | 5.87 | 3.03 | <0.001 | ||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download