Abstract
Objectives
To compare serum vitamin D levels in elderly patients with or without osteoporotic spinal compression fractures (OSCFs) and to identify relationships between the serum vitamin D level and other variables, such as age, bone mineral density (BMD), and bone turnover markers (osteocalcin and C-telopeptide).
Summary of Literature Review
Vitamin D plays a key role in calcium metabolism in the bone tissue. Vitamin D deficiency can lead to decreased BMD and an increased risk of falls and of osteoporotic fractures.
Materials and Methods
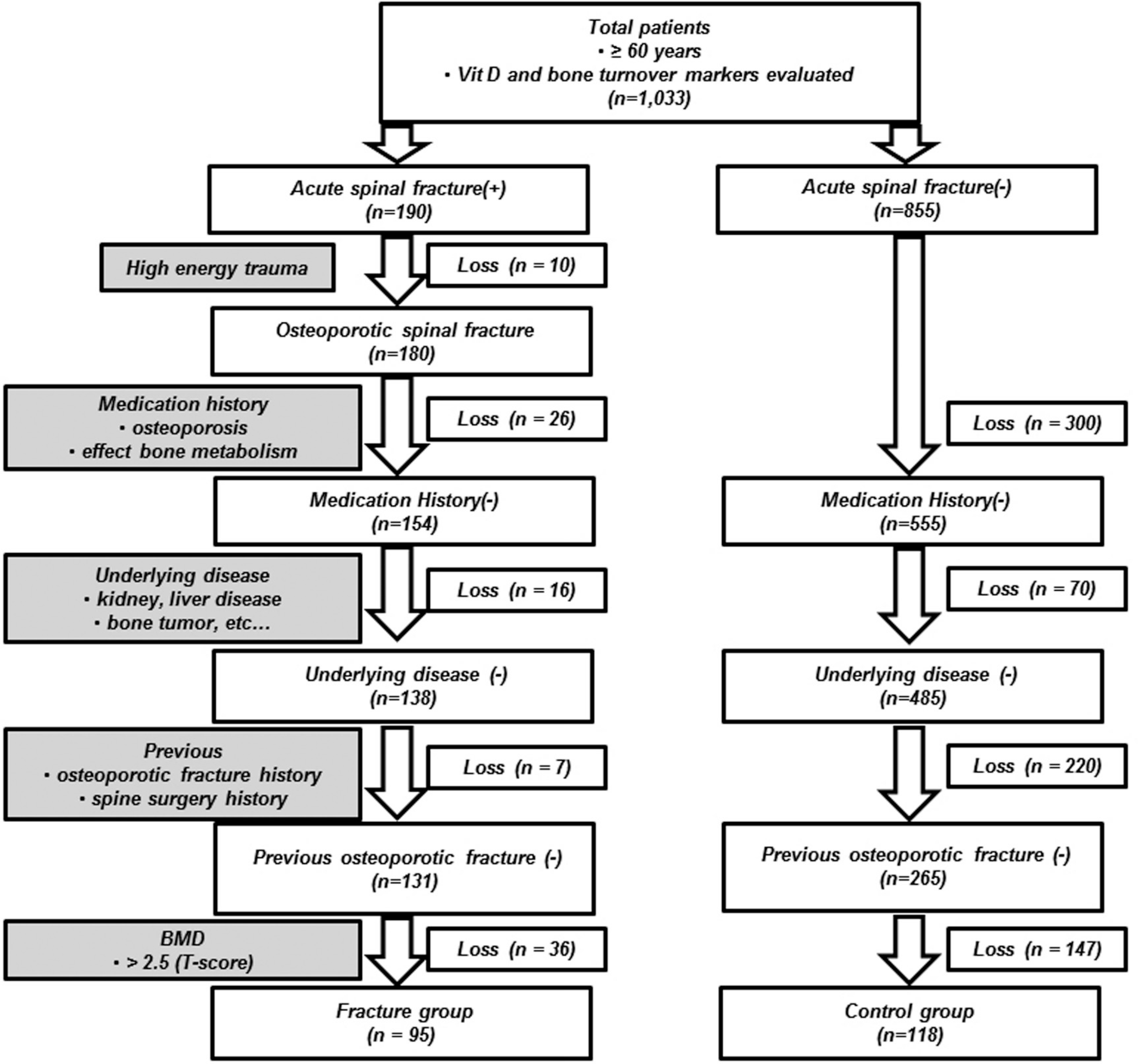
We retrospectively reviewed the medical records of 95 elderly patients (≥60 years) with OSCFs (fracture group) and 118 subjects who had been diagnosed with osteoporosis without OSCFs (control group). Serum vitamin D levels were contrasted between the two groups taking into account other factors such as patient age, sex, and seasonal variations. For all the patients, we also evaluated the correlation between the vitamin D level and the patient age, BMD, and bone turnover markers.
Results
The mean of the serum 25(OH) vitamin D3 levels was significantly lower in the fracture group than in the control group. There were significant differences in the 25(OH) vitamin D3 levels in autumn. In all patients, the mean serum 25(OH) vitamin D3 levels were the highest in autumn and the lowest in spring. Furthermore, the mean serum 25(OH) vitamin D3 levels were significantly correlated with patient age and BMD.
REFERENCES
2. Eisman JA, Kelly PJ, Morrison NA, et al. Peak bone mass and osteoporosis prevention. Osteoporos Int. 1993; 3(Suppl):56–60.
3. Fujiwara S. Epidemiology of osteoporosis and fracture. Clin Calcium. 2004; 14:13–8.
4. Sweet MG, Sweet JM, Jeremiah MP, et al. Diagnosis and treatment of osteoporosis. Am Fam Physician. 2009; 79:193–200.
5. Lips P, Bouillon R, van Schoor NM, et al. Reducing fracture risk with calcium and vitamin D. Clin Endocrinol (Oxf). 2010; 73:277–85.

6. van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE, et al. Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin. 2006; 22:1757–64.

7. Holick MF. The role of vitamin D for bone health and fracture prevention. Curr Osteoporos Rep. 2006; 4:96–102.

8. Sahota O, Masud T, San P, et al. Vitamin D insufficiency increases bone turnover markers and enhances bone loss at the hip in patients with established vertebral osteoporosis. Clin Endocrinol (Oxf). 1999; 51:217–21.

9. Holick MF. Vitamin D requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int. 1998; 8(Suppl):24–9.

10. Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996; 11:337–49.

11. Jang WY, Chung MS, Baek GH, et al. Vitamin D levels in postmenopausal Korean women with a distal radius fracture. Injury. 2012; 43:237–41.

12. Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008; 23:974–9.

13. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation – A meta-analysis of randomized controlled trials. JAMA. 2005; 293:2257–64.
14. Kim DH, Vaccaro AR. Osteoporoptic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006; 6:479–87.
15. Dawson-Hughes B, Heaney RP, Holick MF, et al. Es-timates of optimal vitamin D status. Osteoporos Int. 2005; 16:713–6.

16. No authors listed. Prevention and management of osteoporosis. World Health Organ Tech Rep Ser. 2003; 921:1–164.
17. Moniz C, Dew T, Dixon T. Prevalence of vitamin D inadequacy in osteoporotic hip fracture patients in London. Curr Med Res Opin. 2005; 21:1891–4.

18. Lee WS, Lee SH, Han SB, et al. Vitamin D Inadequacy in Patients with Osteoporotic Hip Fractures. Korean J Bone Metab. 2011; 18:9–14.
19. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the com-munity: randomised double blind controlled trial. BMJ. 2003; 326:469.

20. Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002; 13:257–64.

21. Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003; 88:5766–72.

22. Choi EY. 25 (OH)D status and demographic and lifestyle determinants of 25 (OH)D among Korean adults. Asia Pac J Clin Nutr. 2012; 21:526–35.
23. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardio-vascular disease. Am J Clin Nutr. 2004; 80(Suppl):1678–88.

24. Garnero P, Gineyts E, Riou JP, et al. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–5.

25. Yamaguchi K, Masuhara K, Yamasaki S, et al. Predictive value of a preoperative biochemical bone marker in relation to bone remodeling after cementless total hip arthroplasty. J Clin Densitom. 2003; 6:259–65.

26. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–8.

27. Bjarnason NH, Sarkar S, Duong T, et al. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001; 12:922–30.

28. Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–8.

Table 1.
Demographic data
Table 2.
Mean serum 25(OH)D3 (ng/ml) depending on age and sex
Table 3.
Mean BMD∗ (T-score) and bone turnover markers (ng/ml)
Table 4.
Mean serum 25(OH)D3 (ng/ml) depending on seasonal variations
Table 5.
Correlations between serum 25(OH)D3 (ng/ml) levels and other variab
| Age | Osteocalcin | C-telopeptide | BMD | L-spine | Lt. hip | Rt. hip | |
|---|---|---|---|---|---|---|---|
| r∗ | −0.145 | 0.068 | −0.077 | 0.206 | 0.209 | 0.215 | 0.188 |
| p-value | 0.034 | 0.32 | 0.27 | 0.003 | 0.003 | 0.002 | 0.007 |
Table 6.
Correlations between OSCF and Serum 25(OH)D3 (ng/ml) leve
| OSCF | serum 25(OH)D3 | ||
|---|---|---|---|
| OSCF | r | 1 | −0.335 |
| p | 0.013 | ||
| N | 213 | 213 | |
| r | −0.335 | 1 | |
| serum 25(OH)D3 | p | 0.013 | |
| N | 213 | 213 |
Table 7.
Regression analysis between OSCF and serum 25(OH)D3 (ng/ml) leve




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download