Abstract
Summary of Literature Review
The neurophysiology of pain has been established at the cellular and molecular biology level through many studies. Also, multiple modalities to manage pain have been developed.
Results
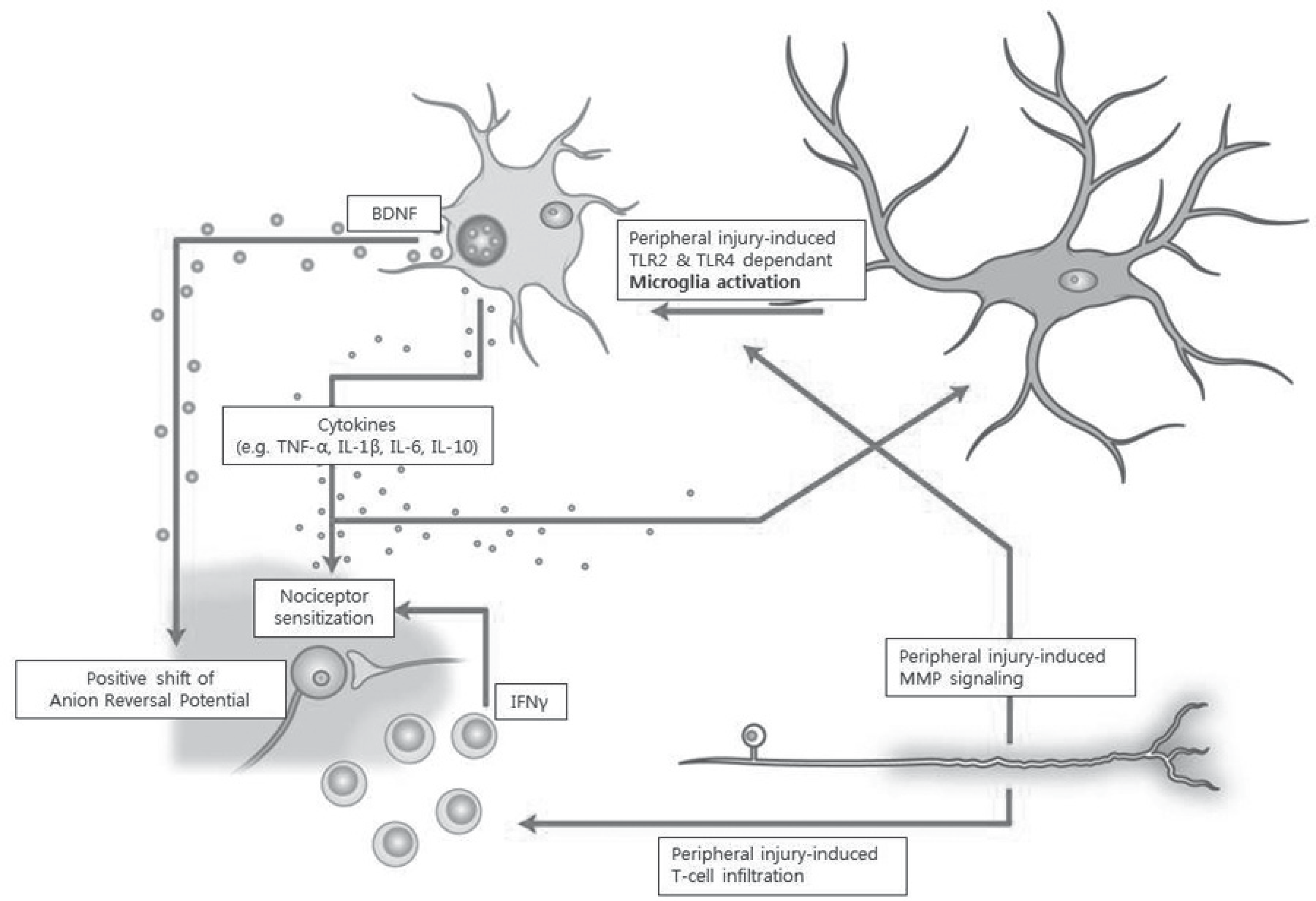
Pain develops by actions of multiple receptors, ion channels and neurotransmitters along the pain pathway. Pathologic states, such as persistent pain, allodynia, and hyperalgesia, arise from alteration of the pain pathway. Especially, neuropathic pain results from nerve injury and its pathology is rather different from the neuroplasty of normal individuals.
Go to : 
REFERENCES
1. Woolf CJ. American Colle�e of P�ysicians; American P�ysiolo�ical Society. Pain: movin� from symptom control toward mec�anism�specific p�armacolo�ic mana�ement. Ann Intern �ed. 2004; 140:441–51.
2. Julius �, Basbaum AI. �olecular mec�anisms of nocicep�tion. Nature. 2001; 413:203–10.
3. von He�n CA, Baron R, Woolf CJ. �econstructin� t�e neuropat�ic pain p�enotype to reveal neural mec�anisms. Neuron. 2012; 73:638–52.
4. Basbaum AI, Bautista ��, Sc�errer �, Julius �. Cellular and molecular mec�anisms of pain. Cell. 2009; 139:267–84.
5. Zeil�ofer HU. T�e �lyciner�ic control of spinal pain pro� cessin�. Cell �ol Life Sci. 2005; 62:2027–35.
6. �illan � J. T�e induction of pain: an inte�rative review. Pro� Neurobiol. 1999; 57:1–164.
7. Boos N, Aebi �. Pat�ways of Spinal Pain in t�e Spinal �is� orders. 1sted.Berlin: Sprin�er Co;2008. .131� 7.
8. �illespie P�, Walker R�. �olecular basis of mec�anosen� sory transduction. Nature. 2001; 413:194–202.
9. Katano T, Nakazawa T, Nakatsuka T, Watanabe �, Yamamoto T, Ito S. Involvement of spinal p�osp�oryla�tion cascade of Tyr1472�NR2B, T�r286�Ca�KII, and Ser831��luR1 in neuropat�ic pain. Neurop�armacolo�y. 2011; 60:609–16.
10. Porreca F, Ossipov � H, �eb�art � F. C�ronic pain and medullary descendin� facilitation. Trends Neurosci. 2002; 25:319–25.
11. Binder A, �ay �, Baron R, et al. Transient receptor poten� tial c�annel polymorp�isms are associated wit� t�e somato� sensory function in neuropat�ic pain patients. PLoS One. 2011; 6:�. 17387.
12. Watabiki T, Kiso T, Kuramoc�i T, et al. Amelioration of neuropat�ic pain by novel transient receptor potential vanil� loid 1 anta�onist AS1928370 in rats wit�out �ypert�ermic effect. J P�armacol �xp T�er. 2011; 336:743–50.
13. Ta L�, Bieber AJ, Carlton S�, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin�induced �eat �yperal�esia in mice. �ol Pain. 2010; 6:15.
14. �anty� PW, Koltzenbur� �, �endell L�, Tive L, S�elton � L. Anta�onism of nerve �rowt� factor�TrkA si�nalin� and t�e relief of pain. Anest�esiolo�y. 2011; 115:189–204.
15. Wu �, Rin�kamp �, �urinson BB, et al. �e�eneration of myelinated efferent fibers induces spontaneous activity in uninjured C�fiber afferents. J Neurosci. 2002; 22:7746–53.
16. He XH, Zan� Y, C�en X, et al. TNF�alp�a contributes to up�re�ulation of Nav1.3 and Nav1.8 in �R� neurons fol� lowin� motor fiber injury. Pain. 2010; 151:266–79.
17. �mery � C, Youn� � T, Berrocoso ��, C�en L, �cNau��� ton PA. HCN2 ion c�annels play a central role in inflam� matory and neuropat�ic pain. Science. 2011; 333:1462–6.
18. Nitzan�Luques A, �evor �, Tal �. �enotype�selective p�enotypic switc� in primary afferent neurons contributes to neuropat�ic pain. Pain. 2011; 152:2413–26.
19. Tan A�, C�an� YW, Z�ao P, Hains BC, Waxman S�. Rac1�re�ulated dendritic spine remodelin� contributes to neuropat�ic pain after perip�eral nerve injury. �xp Neurol. 2011; 232:222–33.
20. Leon� � L, �u �, Speltz�Paiz R, et al. Neuronal loss in t�e rostral ventromedial medulla in a rat model of neuropat�ic pain. J Neurosci. 2011; 31:17028–39.
21. A�rawal S�, Silva C, Tourtellotte WW, Yon� VW. ���PRIN: a novel re�ulator of leukocyte transmi�ration into t�e CNS in multiple sclerosis and experimental autoim� mune encep�alomyelitis. J Neurosci. 2011; 31:669–77.
22. �oulton �A, �lman I, Pendse �, Sc�ma�mann J, Becerra L, Borsook �. Aversion�related circuitry in t�e cerebellum: responses to noxious �eat and unpleasant ima�es. J Neuro� sci. 2011; 31:3795–804.
23. Apkarian AV, Has�mi JA, Baliki � N. Pain and t�e brain: specificity and plasticity of t�e brain in clinical c�ronic pain. Pain. 2011; 152:49–64.
24. Baliki � N, Sc�nitzer TJ, Bauer WR, Apkarian AV. Brain morp�olo�ical si�natures for c�ronic pain. PLoS One. 2011; 6:�. 26010.
25. Baron R, Tolle TR, �ockel U, Brosz �, Freyn�a�en R. A cross�sectional co�ort survey in 2100 patients wit� painful diabetic neuropat�y and post�erpetic neural�ia: �iffer� ences in demo�rap�ic data and sensory symptoms. Pain. 2009; 146:34–40.
26. Ranoux �, Attal N, �orain F, Bou�assira �. Botulinum toxin type A induces direct anal�esic effects in c�ronic neuropat�ic pain. Ann Neurol. 2008; 64:274–83.
27. �dwards RR, Hayt�ornt�waite JA, Tella P, �ax � B, Raja S. Basal �eat pain t�res�olds predict opioid anal�e� sia in patients wit� post�erpetic neural�ia. Anest�esiol� o�y. 2006; 104:1243–8.
Go to : 
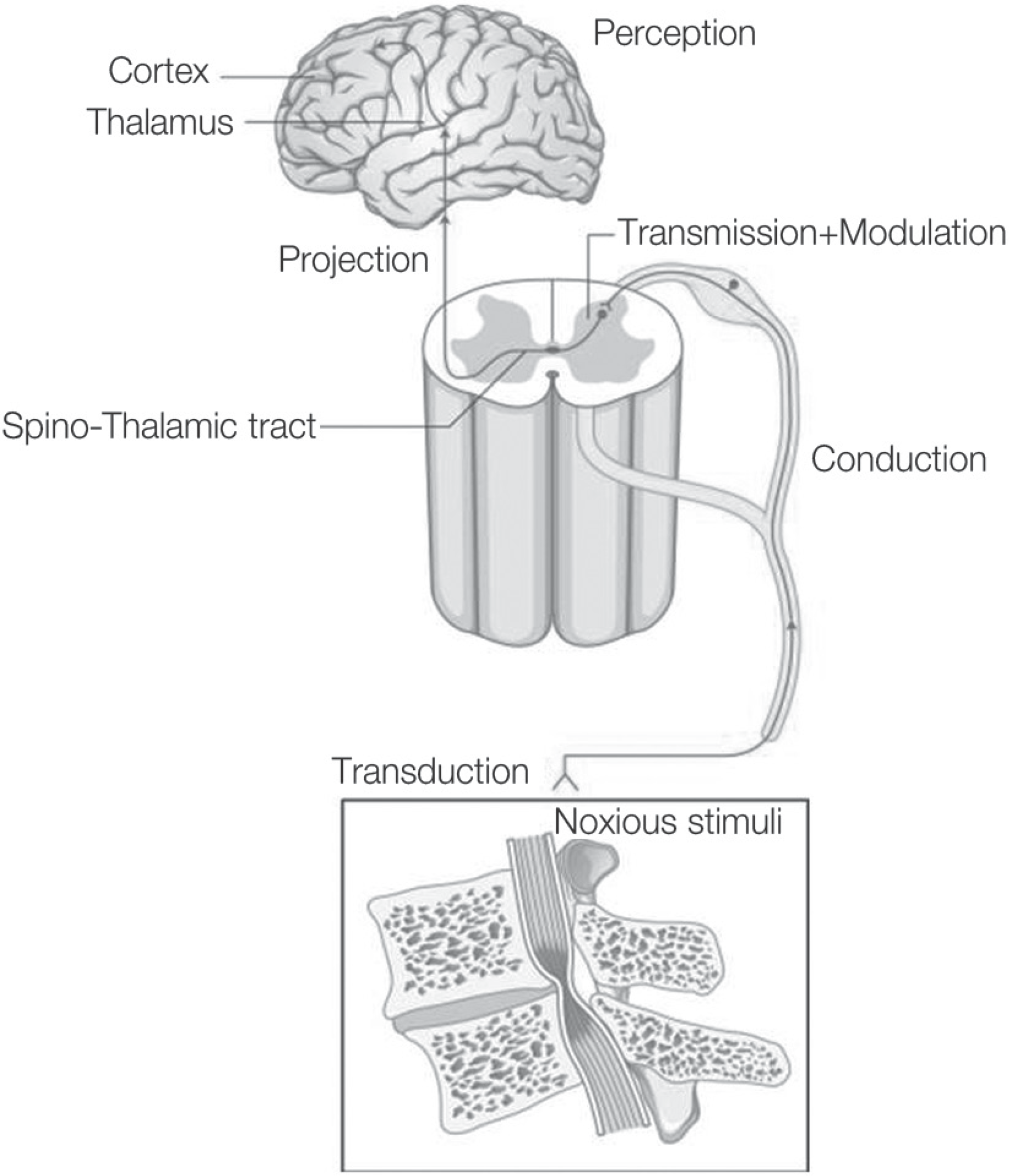 | Fig. 1.Physiologic pathways involved in pain sense are composed of transduction, conduction, transmission, modulation, projection, perception. |
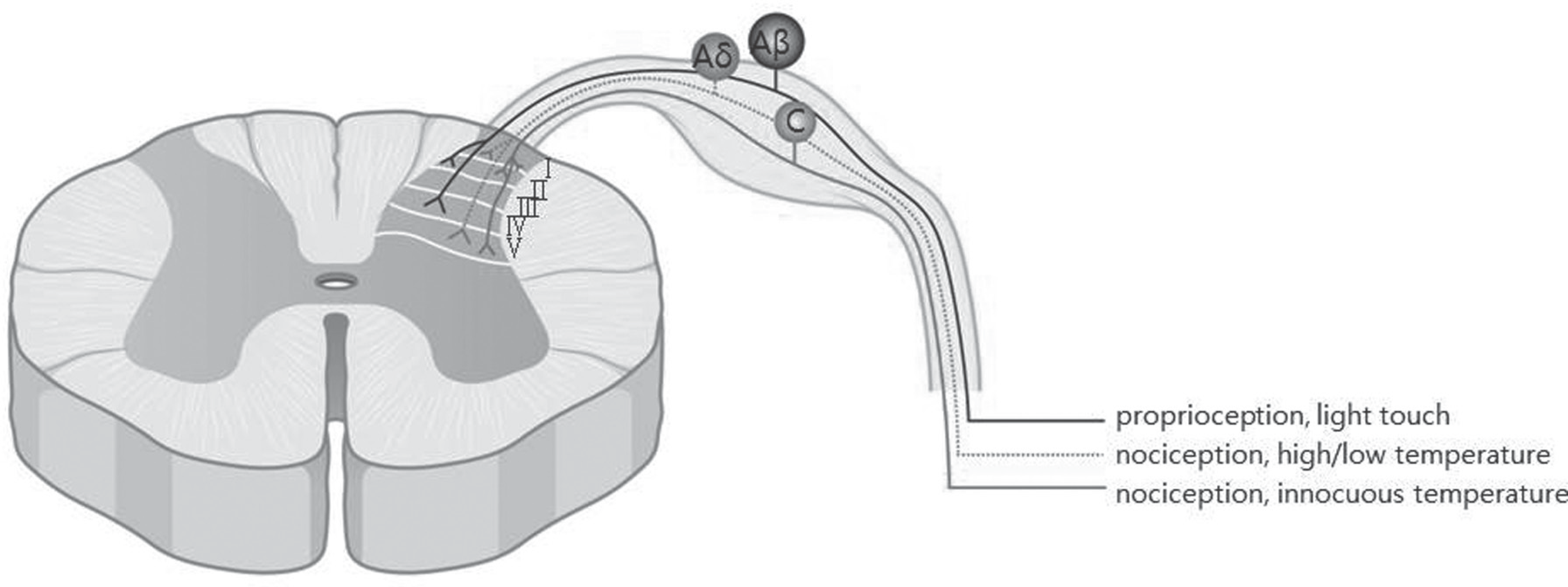 | Fig. 2.Large myelinated low-threshold Aβ afferents terminate in laminae III and IV, lightly myelinated high-threshold Aδ fibers synapse at laminae I and V, and non-myelinated high-threshold C fibers terminate in lamina II but also terminate with some fibers in laminae I and V. |
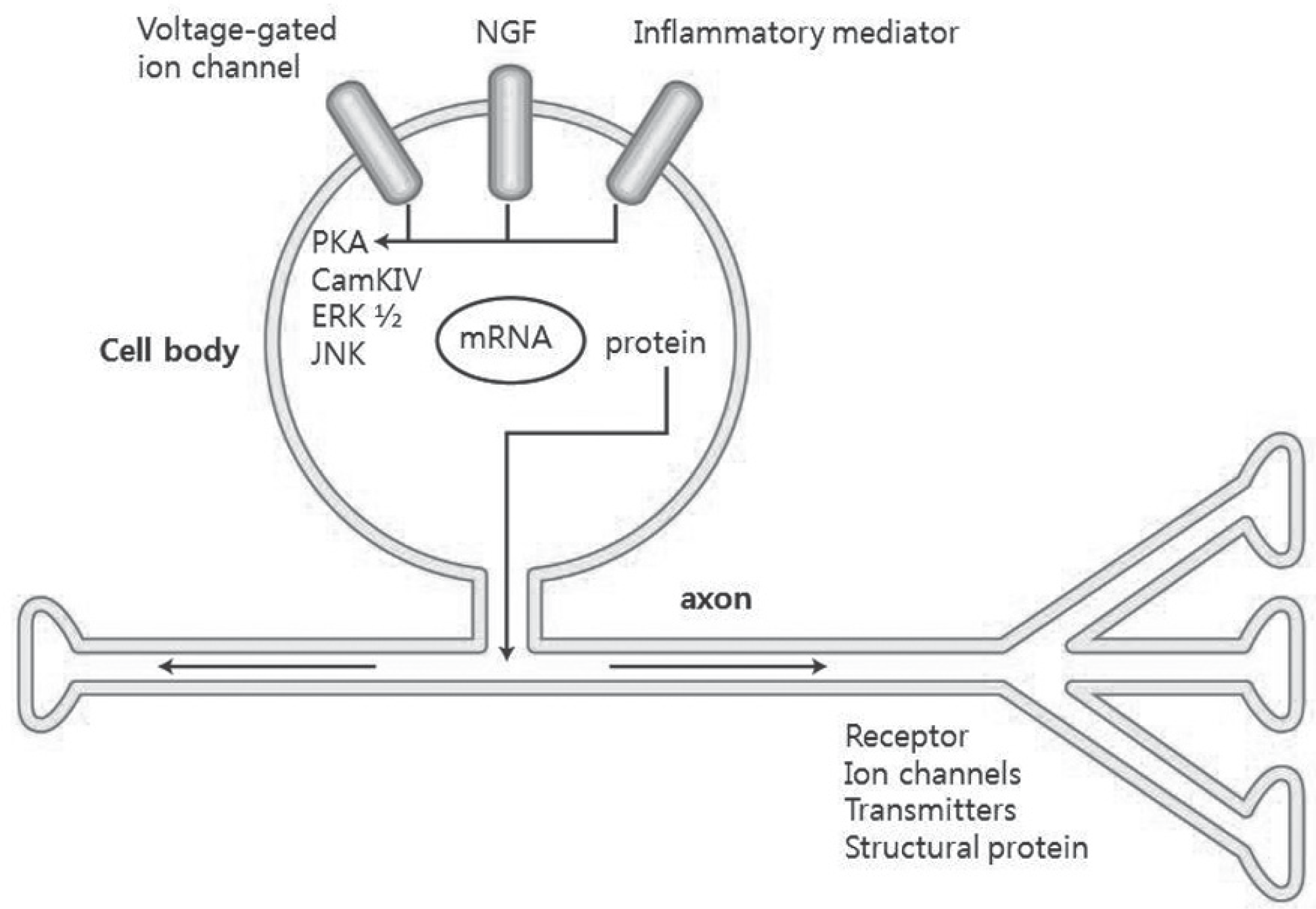 | Fig. 3.Within the DRG, signal transduction cascades are activated, which control the transcription factors that modulate gene expression, leading to changes in the levels of receptors, ion channels, and other structural proteins. |
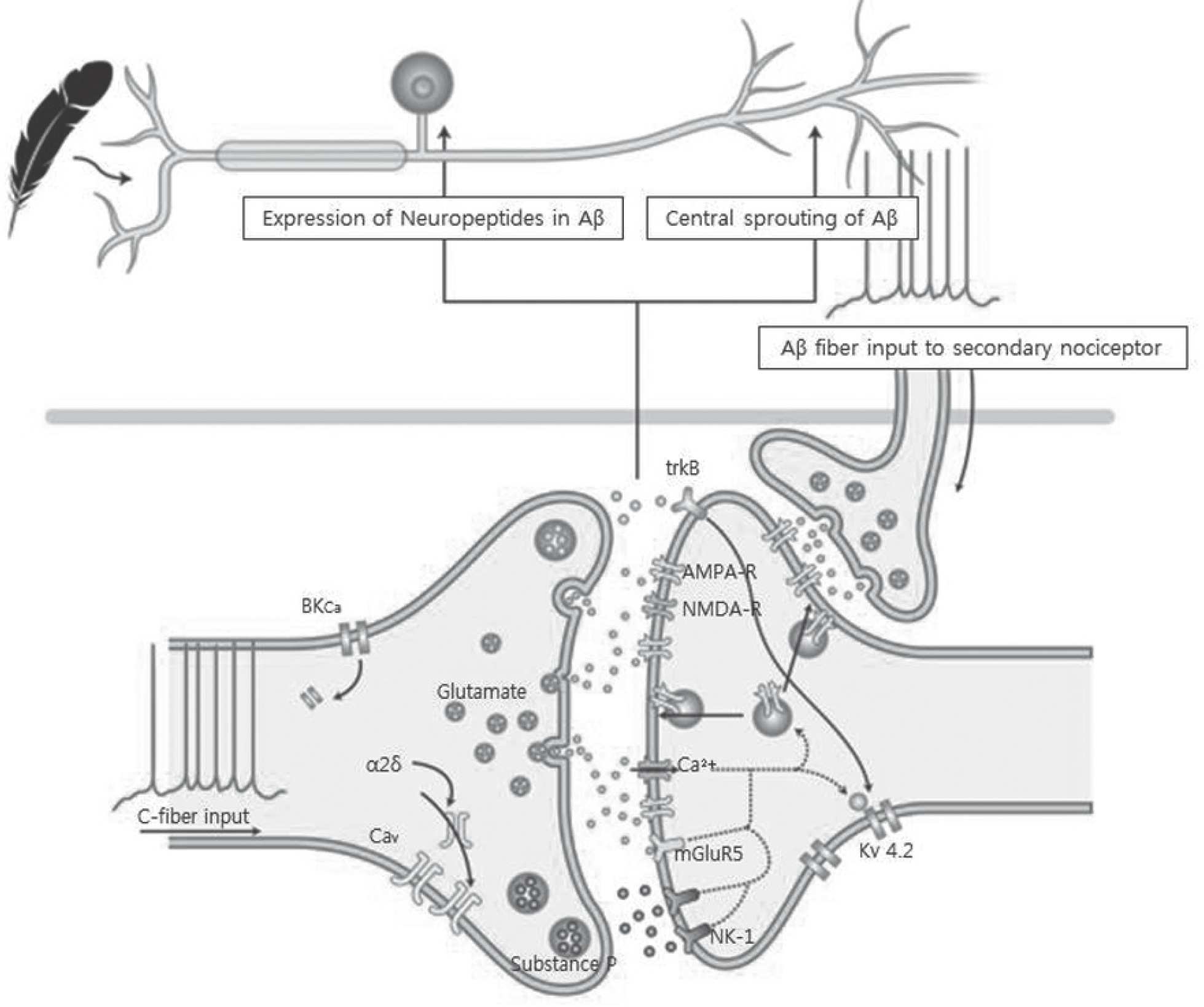 | Fig. 4.Changes due to the nerve injury increase synaptic input from nociceptor and contribute to an amplification of pain, with a reduction in its threshold and a change in its temporal characteristics. A stimulus from C-fiber of nociceptor increases expression of neuropeptides in Aβ and leads to central sprouting of Aβ. Also, receptors for input from Aβ fiber increase in C-fiber. These phenotypic, structural changes cause central sensitization. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download