Abstract
Objectives
The aim of this study was to compare the efficacy of prophylactic antibiotics in spinal surgery for the occurrence of postoperative surgical site infection (SSI) and host immune reactions depending on various administration regimens and protocols.
Summary of Literature Review
The superiority of one regimen or protocol of prophylactic antibiotics over others for SSI in spinal surgery has not been clearly demonstrated. We designed a controlled clinical trial to compare the occurrence of SSI with the changes of hematologic results depending on prophylaxis regimens and protocols.
Materials and Methods
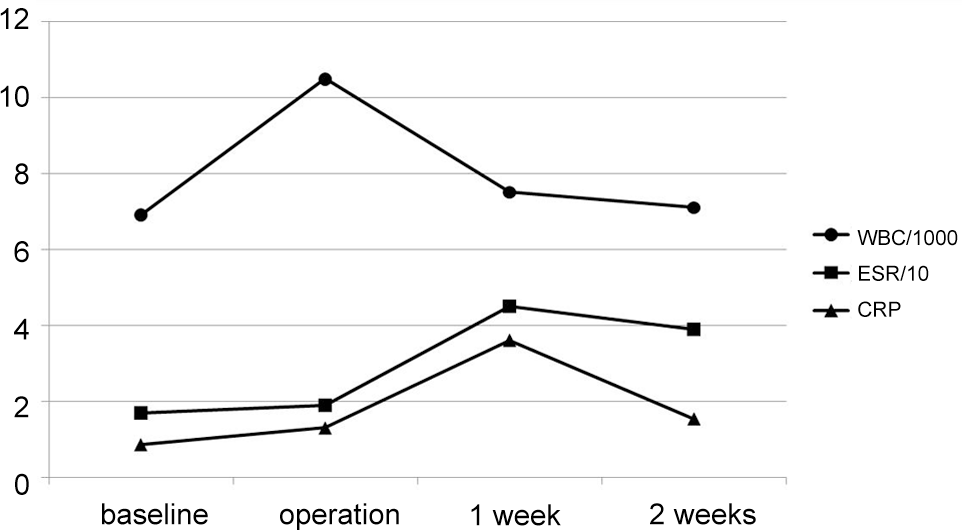
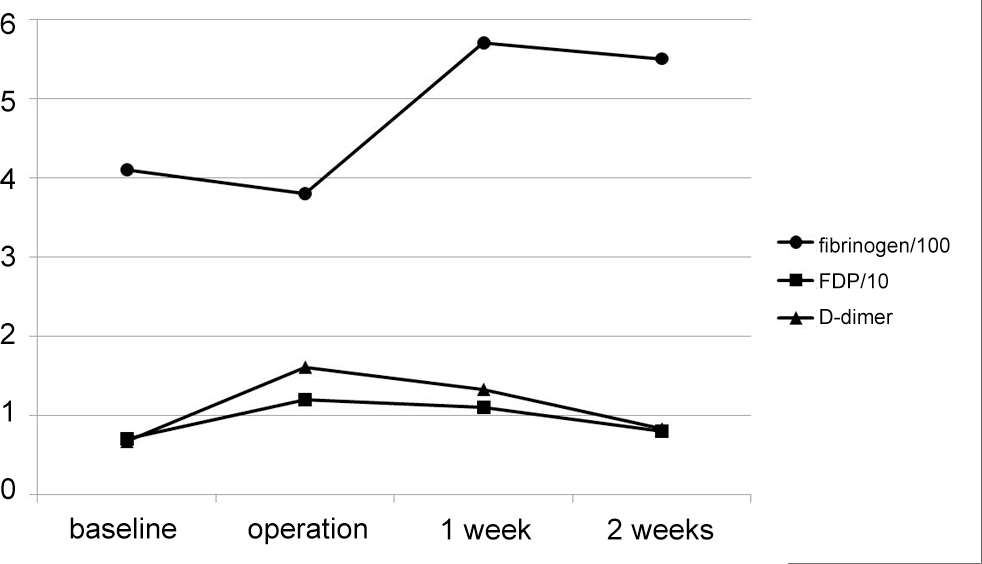
Between January 2007 and February 2011, two hundred consecutive patients who had undergone thoracolumbar/lumbar surgery for degenerative or traumatic disease were included. Postoperative protocol was altered for each group of fifty consecutive patients; 1st generation cephalosporins for 5-days (group A), 2nd generation cephalosporins for 5-days (group B), 1st generation cephalosporins for 3-days (group C), and 2nd generation cephalosporins for 3-days (group D). Preoperative antibiotic prophylaxis was administrated within 1 hour prior to surgical incision with the same trial antibiotics. Intraoperative bacterial culture was performed from the surgical site. The occurrences of SSI were evaluated as either incisional or organ/space SSI. Serial changes in hematologic inflammatory markers (WBC, ESR, CRP) and DIC markers (fibrinogen, FDP, D-dimer) were compared until postoperative 2 weeks.
Results
The study groups were homogeneous regarding age, sex, body mass index, estimated blood loss, diabetes mellitus, smoking, diagnosis, baseline laboratory values, and type of surgery including instrumentation. Overall, 13 cases of incisional SSI (6.5%) and 3 cases (1.5%) of organ/space SSI occurred. There was no difference in the occurrence of incisional and organ/space SSI among the 4 groups (P=0.690, 0.799). Laboratory results revealed that postoperative changes in hematologic inflammatory markers and DIC markers were not influenced by prophylaxis regimens and protocols (all P>0.05).
Go to : 
REFERENCES
1. Ahn DK, Choi DJ, Park HS, Kim TW, Chun TH, Yang JH. Precautions Against Infection Following Posterior Spinal Fusion Based on Types of Infection and Risk Factors. J Korean soc spine surg. 2009; 16:274–84.

2. Kim EH, Song IS. Deep Wound Infection after Lumbar Spine Fusion wigh Pedicualar Screw Fixation. J Korean soc spine surg. 2000; 7:535–43.
3. Song KJ, Song KH, Park YG, Lee KB. Risk Factors of Deep Infection after Thoracic and Lumbar Spinal Arthrodesis. J Korean soc spine surg. 2008; 15:149–56.

4. Shim JI, Kim TS, Lee SJ, et al. Late Infection of Spinal Instrumentation. J Korean soc spine surg. 2000; 7:29–36.
5. Thalgott JS, Cotler HB, Sasso RC, LaRocca H, Gardner V. Postoperative infections in spinal implants. Classification and analysis–a multicenter study. Spine (Phila Pa 1976). 1991; 16:981–4.

6. National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004; 32:470–85.
7. Barker FG 2nd. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002; 51:391–400.
8. Brown EM, Pople IK, de Louvois J, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine (Phila Pa 1976). 2004; 29:938–45.
9. Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008; 90:2–9.

10. Kanayama M, Hashimoto T, Shigenobu K, Oha F, Togawa D. Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline-based antimicrobial prophylaxis in lumbar spine surgery. J Neurosurg Spine. 2007; 6:327–9.

11. Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008; 17:445–50.

12. Petignat C, Francioli P, Harbarth S, et al. Cefuroxime prophylaxis is effective in noninstrumented spine surgery: a double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008; 33:1919–24.
13. Kim B, Moon SH, Moon ES, et al. Antibiotic Microbial and 72-Hour AMP Protocols. Asian Spine J. 2010; 4:71–6.
14. Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009; 34:1422–8.

15. Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005; 30:1460–5.

16. Simpson JM, Silveri CP, Balderston RA, Simeone FA, An HS. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993; 75:1823–9.

17. Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992; 5:277–85.

18. Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998; 11:124–8.
19. Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010; 13:158–64.

20. Sakr Y, Reinhart K, Hagel S, Kientopf M, Brunkhorst F. Antithrombin levels, morbidity, and mortality in a surgical intensive care unit. Anesth Analg. 2007; 105:715–23.

21. Akyildiz HY, Sozuer E, Akcan A, et al. The value of D-di-mer test in the diagnosis of patients with nontraumatic acute abdomen. Ulus Travma Acil Cerrahi Derg. 2010; 16:22–6.
22. Guneysel O, Pirmit S, Karakurt S. Plasma d-dimer levels increase with the severity of community acquired pneumonia. Tuberk Toraks. 2004; 52:341–7.
Go to : 
Figures and Tables%
Table 1.
Patient Demographics
Table 2.
Result of bacterial culture on the surgical site swab
Table 3.
Laboratory Result




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download