Abstract
Objectives
To evaluate and compare the neuroprotective effect of statins, erythropoietin and polyethylene glycol (PEG) after spinal cord injury (SCI).
Summary of Literature Review
There are few comparative studies of pharmacological agents for acute SCI.
Materials and Methods
Forty Sprague Dawley (SD) rats had a spinal cord injury at T9/10 using an Ohio State University (OSU) impactor. The animals were randomized to receive one of the following; simvastatin, erythropoietin, PEG or saline. A behavioral outcome assessment was performed on days 2, 4 and 7, and then every week using the Basso, Bresnahan, and Beattie (BBB) score and subscore. The animals were sacrificed at the end of 6 weeks and histologic assessment was performed to measure the areas of white and gray matter.
Results
For the animals treated with simvastatin, erythropoietin, PEG and saline, the mean BBB scores at 6 weeks post-injury were 13.2±0.1, 11.7±0.4, 13.3±0.3, and 11.4±0.2, and the BBB subscores were 9.2±1.1, 5.0±1.3, 9.1±1.1, 4.4±1.2, respectively. The BBB scores and BBB subscores were significantly higher in simvastain and PEG-treated animals (p<0.05). The areas of white matter at the lesion epicenter were 0.78±0.05mm2, 0.46±0.04 mm2, 0.68±0.15 mm2, and 0.41±0.04mm2 in the simvastatin, erythropoietin, PEG and saline groups, respectively. The simvastatin and PEG-treated animals showed increased sparing of the white matter at the injury epicenter and at 0.2mm rostral and 0.4mm caudal(p<0.05).
Go to : 
REFERENCES
1.Nobunaga AI., Go BK., Karunas RB. Recent demographic and injury trends in people served by the Model Spinal Cord Injury Care Systems. Arch Phys Med Rehabil. 1999. 80:1372–82.

2.Davies SJ., Goucher DR., Doller C., Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999. 19:5810–22.

3.Fournier AE., Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001. 11:89–94.

4.Kwon BK., Tetzlaff W., Grauer JN., Beiner J., Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004. 4:451–64.

5.Bracken MB., Collins WF., Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984. 251:45–52.

6.Bracken MB., Shepard MJ., Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990. 322:1405–11.
7.Bracken MB., Shepard MJ., Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997. 277:1597–604.

8.Hall ED., Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004. 1:80–100.

9.Pannu R., Barbosa E., Singh AK., Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005. 79:340–50.

10.Stepien K., Tomaszewski M., Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005. 57:561–9.
11.Celik M., Gokmen N., Erbayraktar S, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002. 99:2258–63.

12.Luo J., Shi R. Polyethylene glycol inhibits apoptotic cell death following traumatic spinal cord injury. Brain Res. 2007. 1155:10–6.

13.Kwon BK., Roy J., Lee JH, et al. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective ther-apy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma. 2009. 26:1379–93.

14.Kwon BK., Sekhon LH., Fehlings MG. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine (Phila Pa 1976). 2010. 35:S263–70.

15.Basso DM., Beattie MS., Bresnahan JC. A sensitive and reli-able locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.

16.Popovich PG., Guan Z., Wei P., Huitinga I., van Rooijen N., Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999. 158:351–65.

17.Gorio A., Gokmen N., Erbayraktar S, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002. 99:9450–5.

18.Wells JE., Hurlbert RJ., Fehlings MG., Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003. 126:1628–37.

19.Zacco A., Togo J., Spence K, et al. 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003. 23:11104–11.

20.Borgens RB., Bohnert D. Rapid recovery from spinal cord injury after subcutaneously administered polyethylene glycol. J Neurosci Res. 2001. 66:1179–86.

21.Borgens RB., Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000. 14:27–35.

Go to : 
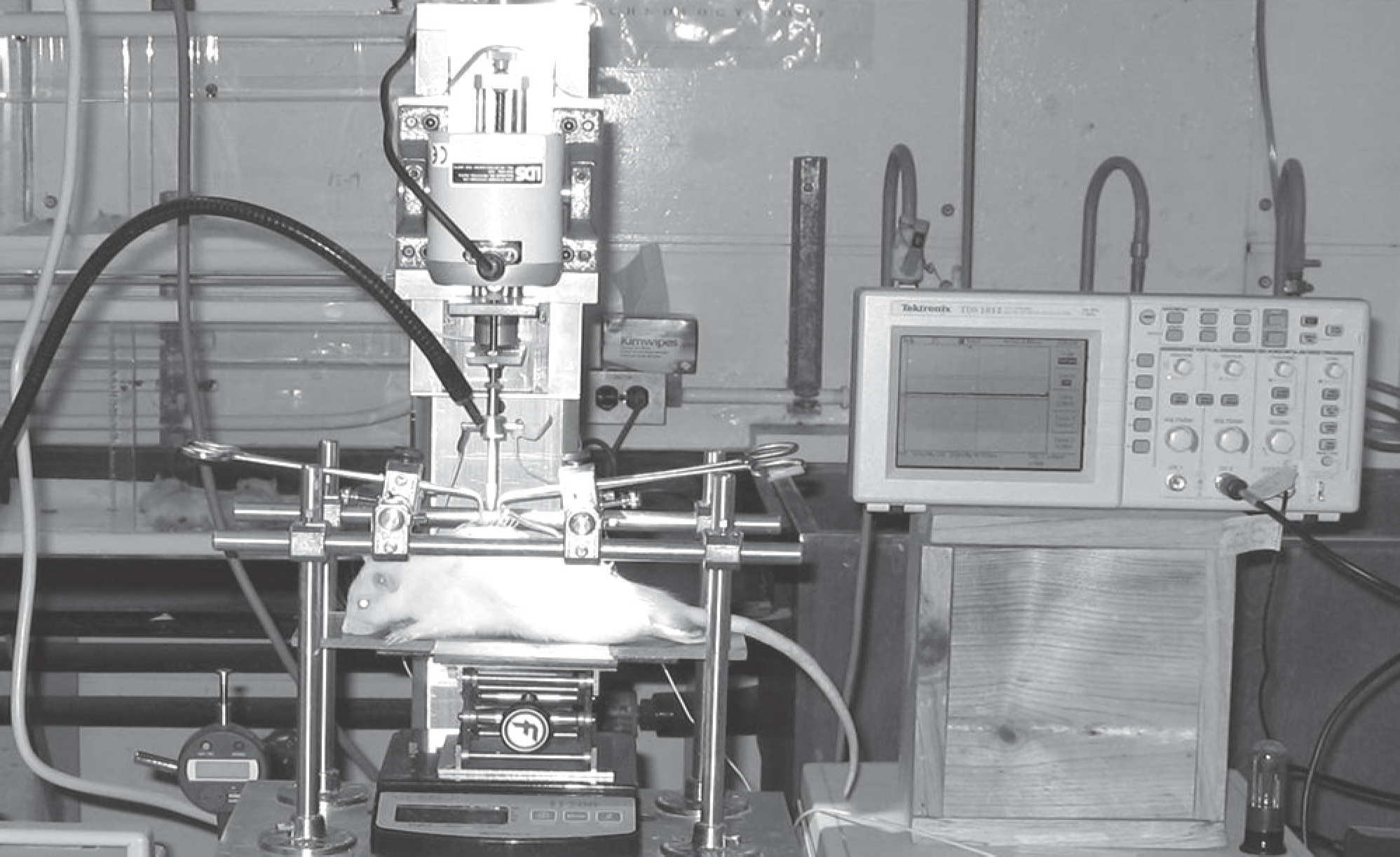 | Fig. 1.Ohio State University (OSU) Impactor. A laminectomy (T9-10) was performed, and the bases of the adjacent spinous processes were secured with modified Allis clamps. The impactor was then triggered to deliver mechanical injury. Animal model of acute spinal cord contusion was made. |
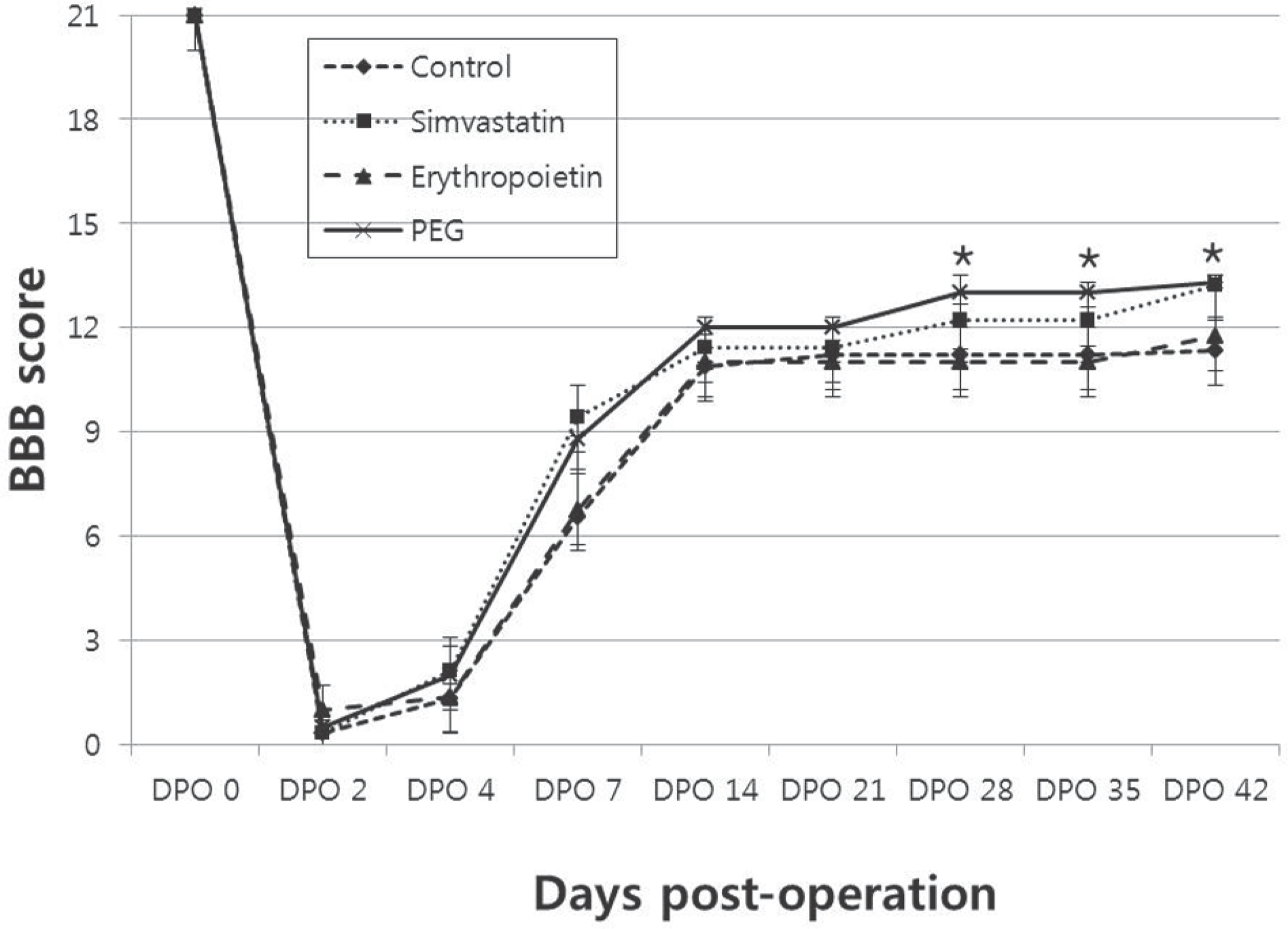 | Fig. 2.BBB score. Simvastatin and PEG-treated animals showed im-proved open-field locomotor (BBB) scores compared with erythropoietin and saline-treated control animals (∗, p<0.05). |
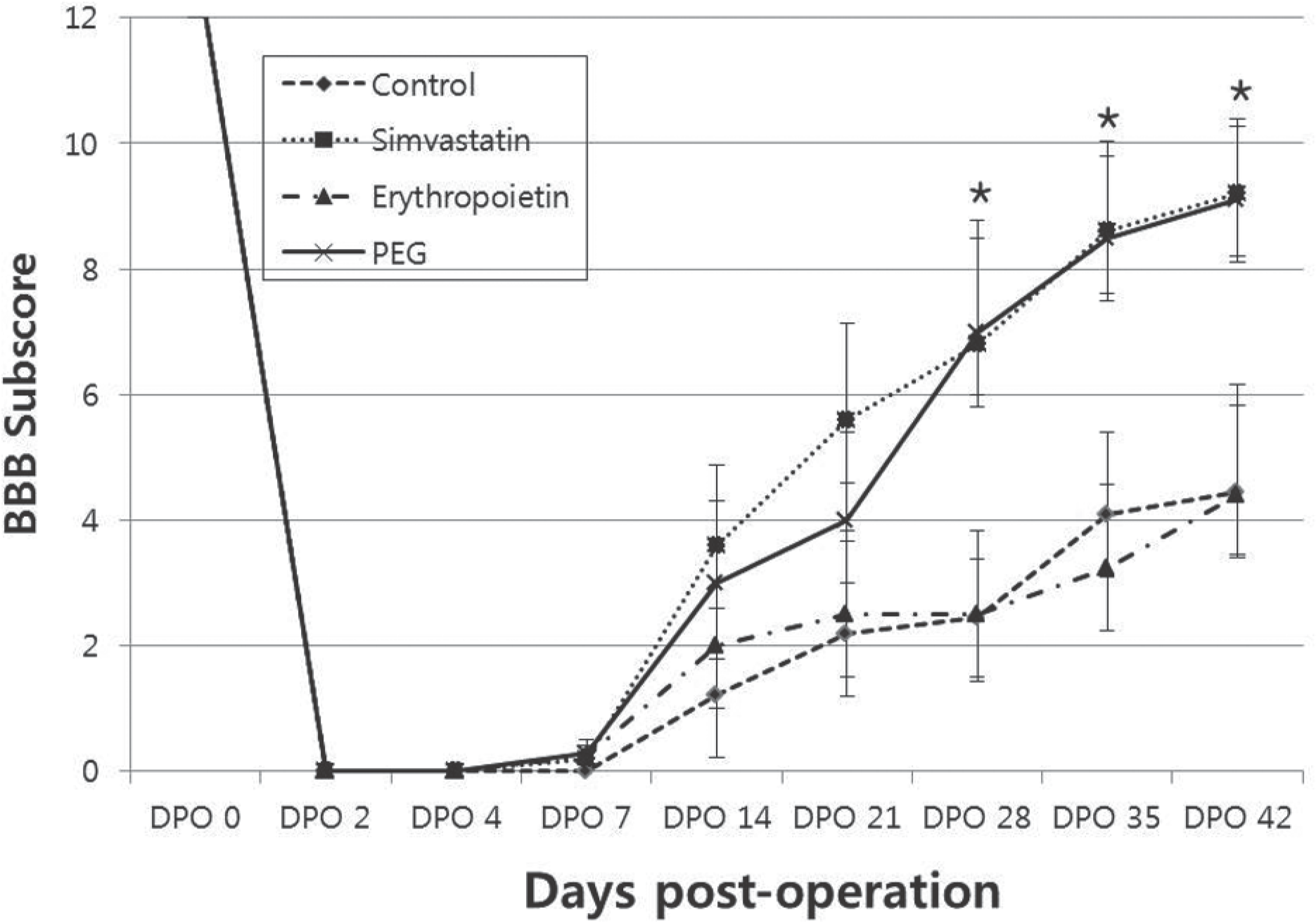 | Fig. 3.BBB subscore. Simvastatin and PEG-treated animals showed im-proved BBB subscores compared with erythropoietin and saline-treated control animals (∗, p<0.05). |
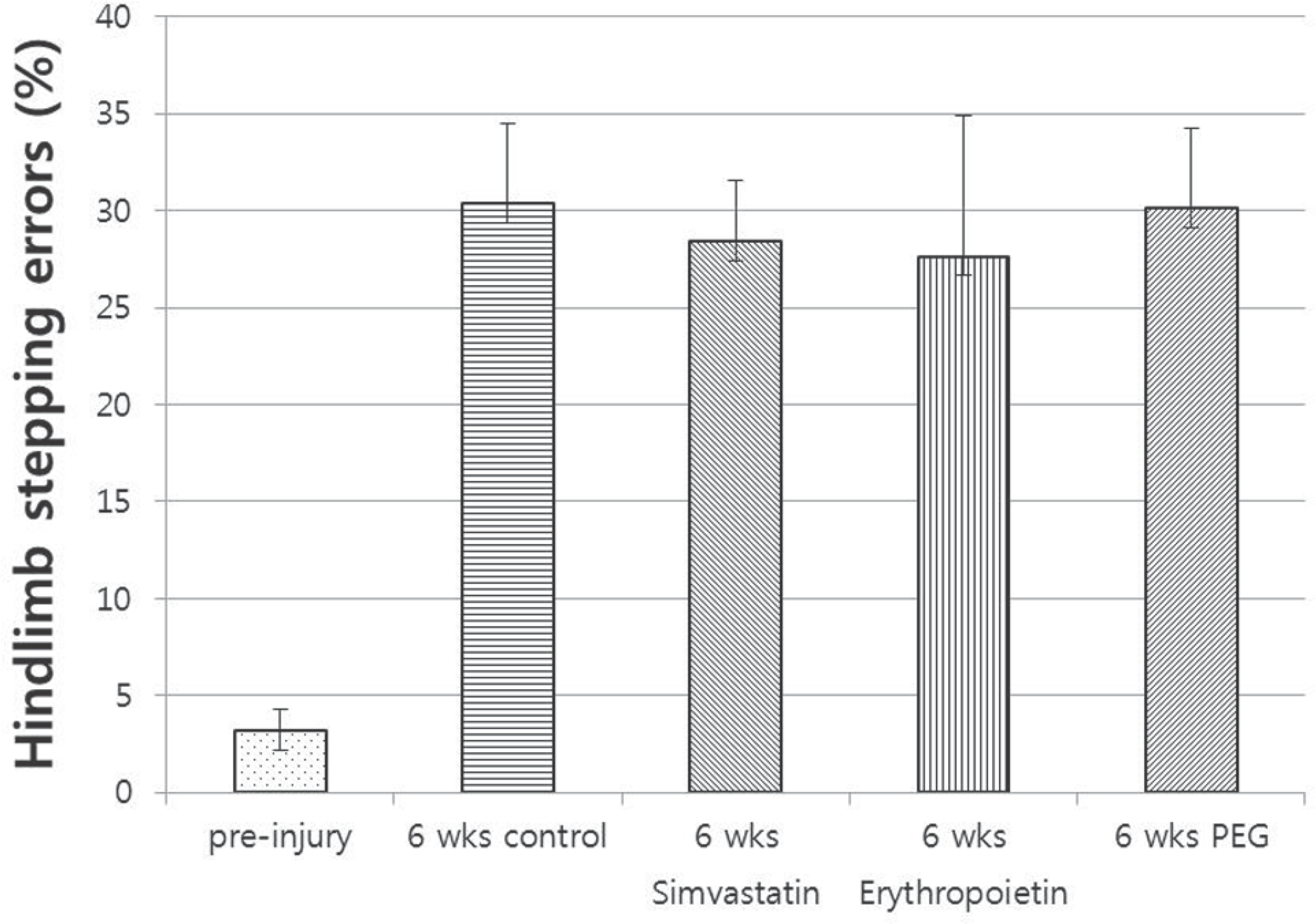 | Fig. 4.Horizontal ladder test. Comparing with preinjury, four groups showed increased hindlimb stepping errors. There was no difference among the four groups after 6 weeks post injury (p>0.05). |
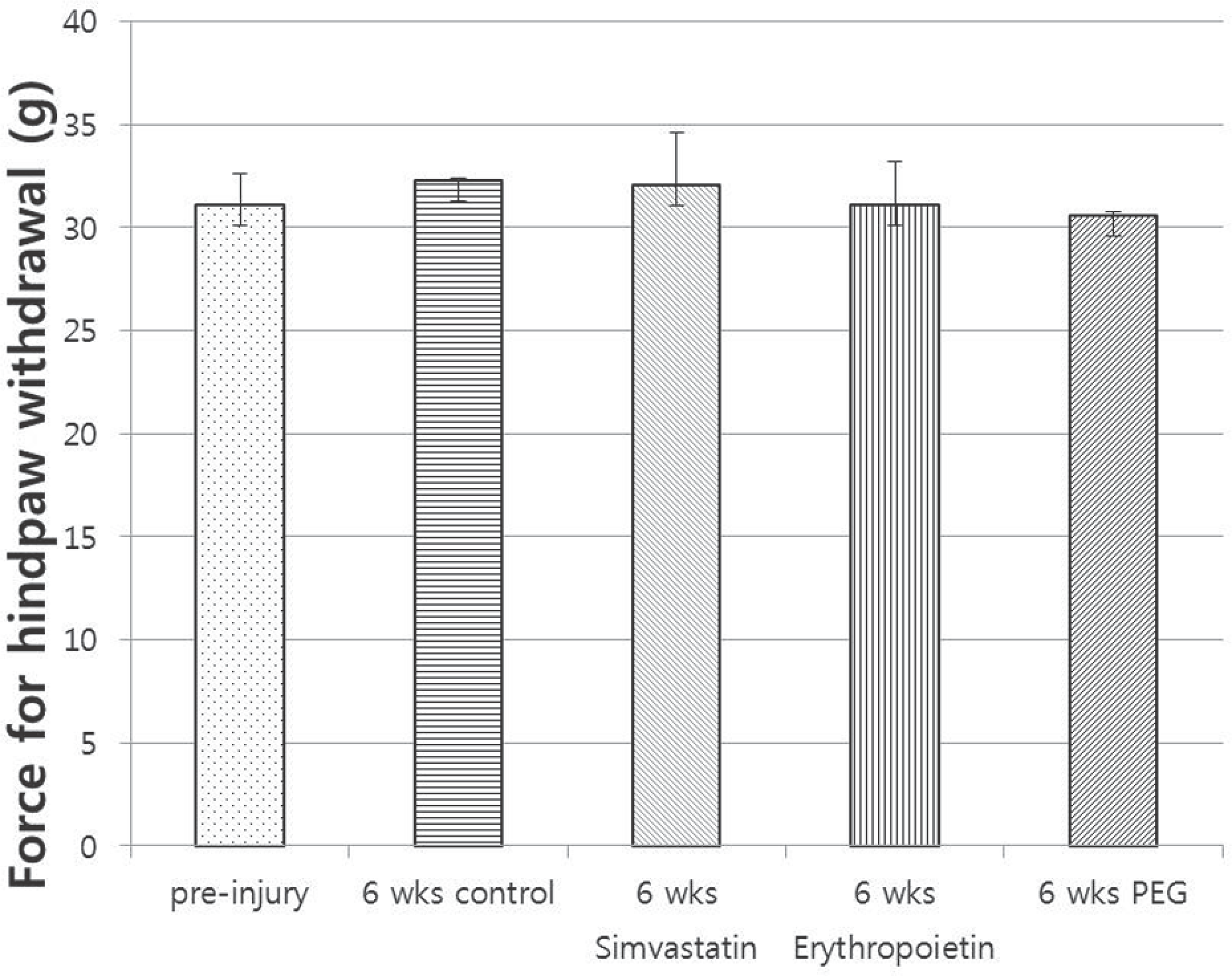 | Fig. 5.Pinprick sensory test. There was no difference among the four groups after 6 weeks post injury (p>0.05). |
 | Fig. 6.Histology assessment. There was no significant difference of gray matter sparing among the four groups. The simvastatin and PEG-treated ani-mals showed increased sparing of the white matter at the injury epicenter and at 0.2mm rostral and 0.4mm caudal(∗, p<0.05). |
Table 1.
Biomechanical parameters of the contusion injury. There were no significant differences among the four groups with respect to the peak force of the injury and the displacement of the impactor tip.
| Groups | Force (kdynes) | Displacement(mm) | n |
|---|---|---|---|
| Simvastatin | 227 ± 5.24 | 1.46 ± 0.03 | 10 |
| Erythropoietin | 225 ± 4.72 | 1.46 ± 0.03 | 10 |
| Polyethylene glycol | 226 ± 6.74 | 1.46 ± 0.03 | 10 |
| Control | 226 ± 6.32 | 1.46 ± 0.03 | 10 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download