Abstract
Objectives
To compare the radiological and clinical results between cage and cancellous allograft mixed with bone marrow for monosegmental instrumented posterior lumbar interbody fusion (PLIF).
Summary of the Literature Review
Allograft has potential problems, such as delayed union. Autologous bone marrow provides for improving the capability of bone induction with allograft. There are rare reports on PLIF using allograft mixed with autologous bone marrow.
Materials and Methods
Monosegmental instrumented PLIF was performed on 51 patients who had lumbar degenerative disease, cage for 28 patients (cage group) and allograft mixed with bone marrow for 23 patients (allograft group). The clinical and radiological results in each group were compared.
Results
The mean follow-up was 45 (30 - 111) months. At the final follow up, there was no significant difference between the cage group and the allograft group in the Korean Version Oswestry Disability Index (p=0.72) and Visual Analogue Score for back pain (p=0.54) and radiating pain to the leg (p=0.26). The radiological fusion rate was 92.8% in the cage group, and 82.6% in the allograft group (p=0.02). At the last follow up, disc height was decreased to 1.5±0.8 mm of the cage group, and 3.0±1.5 mm of the allograft group (p=0.0001).
Go to : 
REFERENCES
1.Enker P., Steffee AD. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994. 300:90–101.

2.Lin PM. Posterior lumbar interbody fusion technique: com-plications and pitfalls. Clin Orthop Relat Res. 1985. 193:90–102.
3.Verlooy J., De Smedt K., Selosse P. Failure of a modified posterior lumbar interbody fusion technique to produce ad-equate pain relief in isthmic spondylolytic grade 1 spondy-lolisthesis patients: A prospective study of 20 patients. Spine (Phila Pa 1976). 1993. 18:1491–5.
4.Brantigan JW., Steffe AD., Geiger JM. A carbon fiber implant to aid interbody lumbar fusion. Mechanical testing. Spine (Phila Pa 1976). 1991. 16(6 Suppl):S277–82.
5.Okuyama K., Kido T., Unoki E., Chiba M. PLIF with a titanium cage and excised facet joint bone for degenerative spondylolisthesis in augmentation with a pedicle screw. J Spinal Disord Tech. 2007. 20:53–9.
6.Miura Y., Imagama S., Yoda M., Mitsuguchi H., Kachi H. Is local bone viable as a source of bone graft in posterior lumbar interbody fusion? Spine (Phila Pa 1976). 2003. 28:2386–9.

7.Jorgenson SS., Lowe TG., France J., Sabin J. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patients: A minimum of 1-year follow-up in 144patients. Spine (Phila Pa 1976). 1994. 19:2048–53.
8.Aurori BF., Weierman RJ., Lowell HA., Nadell Cl., Parsons JR. Pseudoarthrosis after spinal fusion for scoliosis: A comparison of autogenous and allogenic bone grafts. Clin Orthop Relat Res. 1985. 199:153–8.
9.An HS., Lynch K., Toth J. Prospective Comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995. 8:131–5.
10.Dodd CA., Fergusson CM., Freedman L., Houghton GR., Thomas D. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg Br. 1988. 70:431–4.

11.Bridwell KH., O'Brien MF., Lenke LG., Baldus C., Blanke K. Posterior spinal fusion supplement with only allograft bone in paralytic scoliosis. Does it work? Spine (Phila Pa 1976). 1994. 19:2658–66.
12.Fabry G. Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991. 11:465–8.

13.Gibson S., McLeod I., Wardlaw D., Urbaniak S. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine (Phila Pa 1976). 2002. 27:1599–603.
14.Malloy KM., Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res. 2002. 394:27–38.

15.Connolly JF., Guse R., Tiedeman J., Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991. 266:259–70.

16.Connolly JF. Injectable bone marrow preparations to stim-ulate osteogenic repair. Clin Orthop Relat Res. 1995. 313:8–18.
17.Seitz WH Jr., Froimson AI., Leb RB. Autogenous bone marrow and allograft replacement of bone defects in the hand and upper extremities. J Orthop Trauma. 1992. 6:36–42.
18.Lane JM., Yasko AW., Tomin E, et al. Bone marrow and recombinant human bone morphogenetic protein-2 in os-seous repair. Clin Orthop Relat Res. 1999. 361:216–27.

19.Vaccaro AR., Chiba K., Heller JG, et al. Bone grafting alter-natives in spinal surgery. Spine J. 2002. 2:206–15.

20.Jeon CH., Kim DJ., Lee HM, et al. Cross-cultural adaptation of the korean version of the oswewtry disability index(ODI). J Korean Soc Spine Surg. 2005. 12:146–52.
21.Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976). 1994. 19:1271–9.

22.Slosar PJ., Josey R., Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine (Phila Pa 1976). 2007. 7:301–7.

23.Vaidya R., Weir R., Sethi A., Meisterling S., Hakeos W., Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007. 89:342–5.

24.Connolly JF., Shindell R. Percutaneous marrow injection for an ununited tibia. Nebr Med J. 1986. 71:105–7.
25.Scoff HD. Bone marrow/allograft component therapy: A clinical trial. Am J Orthop (Belle Mead NJ). 1995. 24:40–7.
26.Curylo LJ., Johnstone B., Petersilge CA., Janicki JA., Yoo JU. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine (Phila Pa 1976). 1999. 24:434–8.

27.Kumar A., Kozak JA., Doherty BJ., Dickson JH. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine (Phila Pa 1976). 1993. 18:2393–400.

28.Jung KH., Jang JS., Choi YR. Core decompression and Im-paction bone graft in osteonecrosis of the femoral head. J Korean Hip Soc. 2005. 17:223–9.
Go to : 
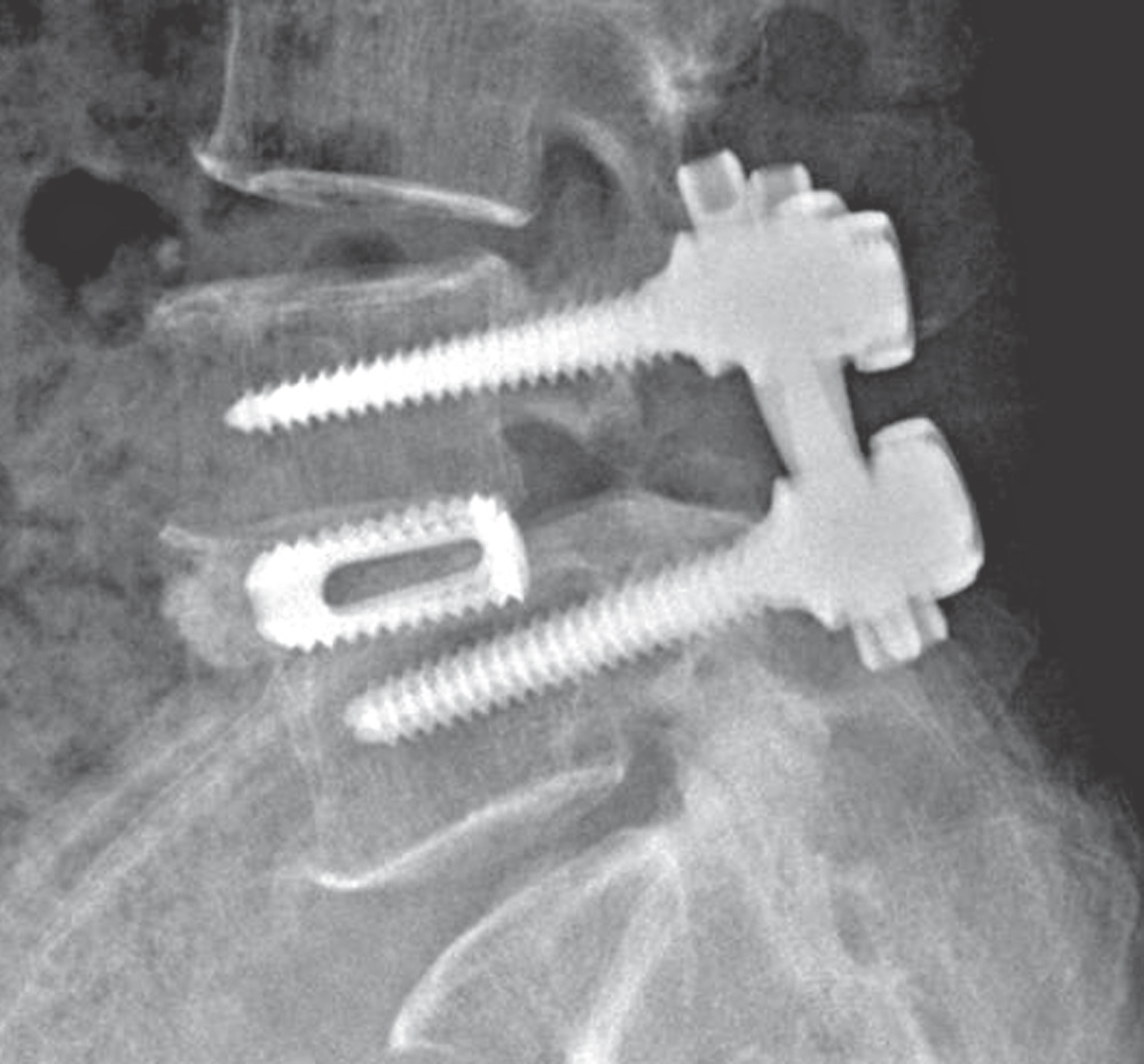 | Fig. 1.Case of cage group. A 63 year old female patient who had spon-dylolisthesis L4 on L5 was undergone posterior lumbar interbody fusion with cage and local bone. |
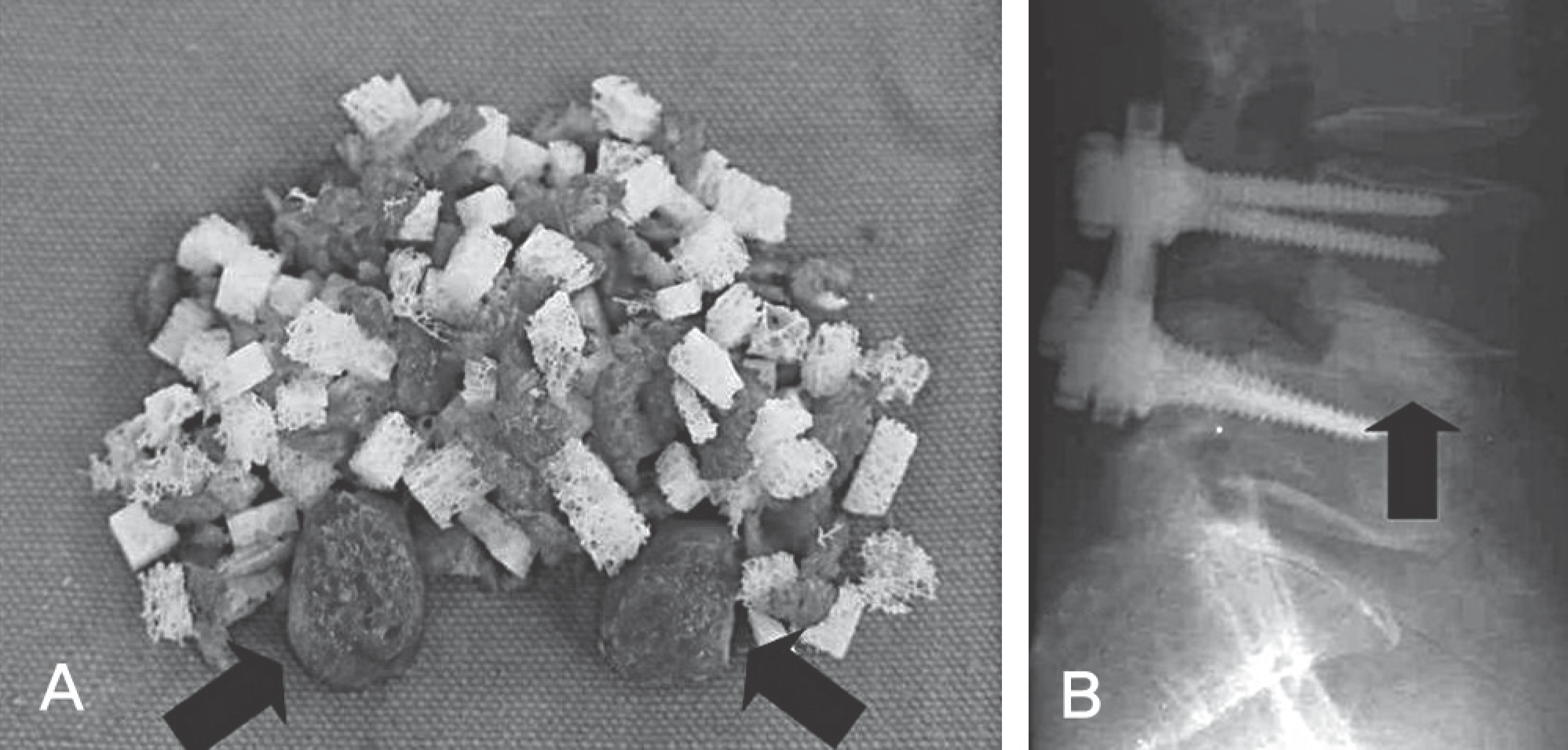 | Fig. 2.Case of allograft group. Photograph of the allograft and bone block (A), this bone block (arrow) that was harvested from facet joint could be provided not only mechanical stability of the disc space as a cage but also prevention from extrusion of mosellized graft. Postoperative lateral radiograph (B). |
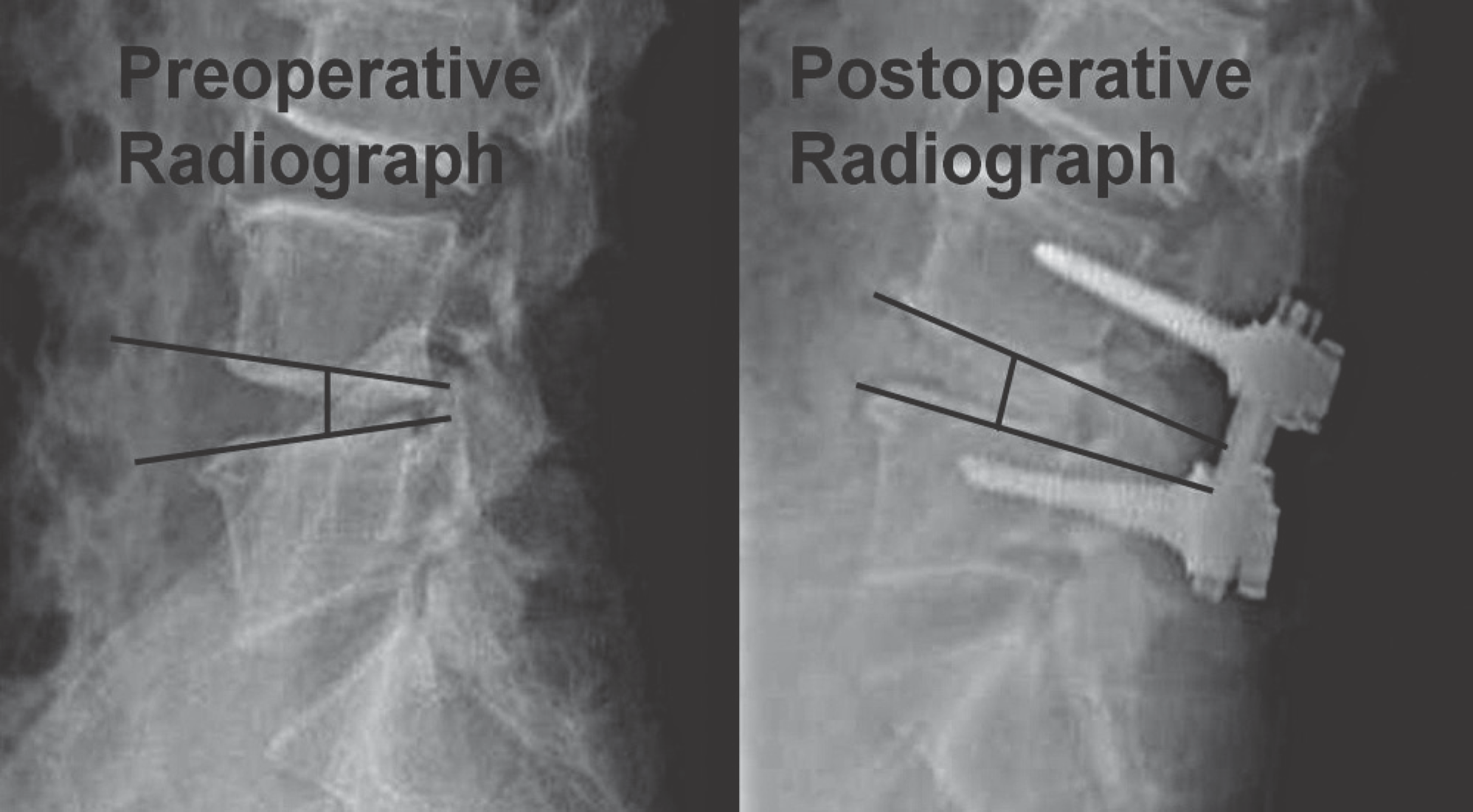 | Fig. 3.The disc height was measured at the preoperative, postoperative and follow-up lateral radiograph. |
 | Fig. 4.Revision for one case of allograft group. At 6-months follow-up, radiograph shows loss of disc height and absorption of the allograft (B). Histological finding reveals inflammation around dead allograft (C). After radical curettages, two cages filled with autograft were inserted (D) and at 40-months follow-up, computed tomographs show well bony fusion and loss of disc height (E). |
 | Fig. 5.At 36 months follow-up, radiograph (A) and MRI (B) didn't achieved bony fusion of the allograft exactly. But at 54 months follow-up, radiograph revealed well bony fusion and maintenance of the disc height. |
Table 1.
Brantigan and Steffee classification for radiologic union
Table 2.
The Clinical Results and Radiological Fusion Rate between two Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download