Abstract
Study Design
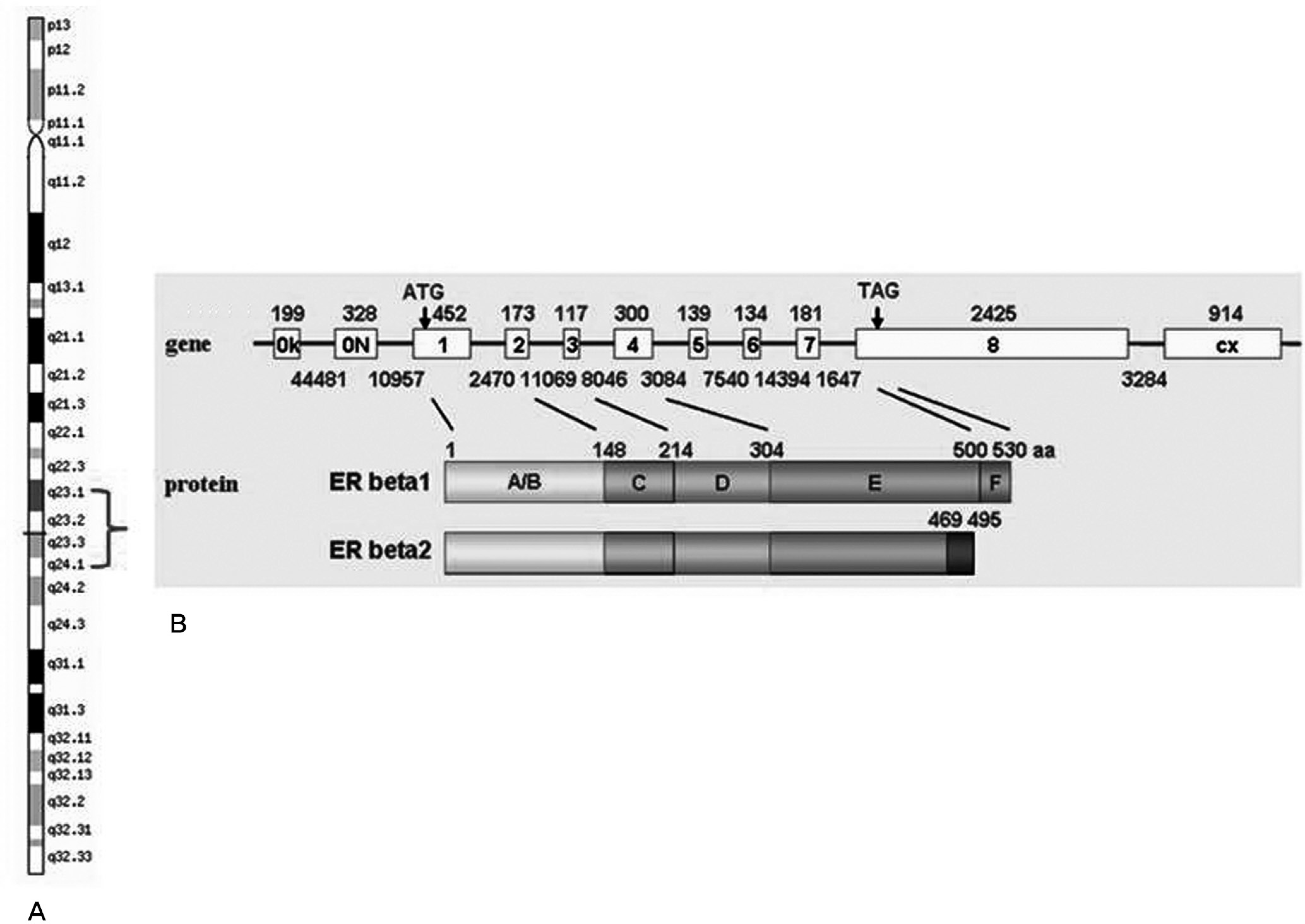
Genetic screening of the estrogen receptor 2 (ESR2) genes in patients with ossification of the posterior longitudinal ligament (OPLL).
Objective
We studied the relationships between ESR2 gene polymorphisms and OPLL to understand the pathophysiology of OPLL.
Summary of Literature Review
The OPLL has a strong genetic component. Several familial surveys and human leukocyte antigen (HLA) haplotype studies reveal that genetic background is an important component in the occurrence of OPLL and a large number of gene analysis studies were utilized to clarify the susceptible gene for OPLL, including COL11A2, BMP-2, TNF-α, NPPS, leptin receptor, transforming growth factor (TGF)-β, Retinoic X receptor, ER, IL-1, PTH, and VDR have been performed.
Materials and Method
Genomic deoxyribonucleic acid (DNA) samples obtained from 164 patients (93 men and 71 women) with OPLL and 219 control subjects, without the disease (105 men and 114 women) were amplified by polymerase chain reaction, and polymorphism genotypes were determined by the restriction endonuclease digestion. The distribution of genotypes was compared between the patients with the disease and the control subjects.
Results
The polymorphism of ESR2 [rs1256049, exon6, Val328Val, p=0.018, odd ratio (OR)=2.41, 95 confidence interval (CI)=1.15-5.02 in the recessive model] only showed statistically significant association between the control and the OPLL groups. The rest SNPs of ESR2 did not show any significant differences between the control and the OPLL groups.
Go to : 
REFERENCES
1.Ono K., Yonenobu K., Miyamoto S., Okada K. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin Orthop Relat Res. 1999. 359:18–26.

2.Taketomi E., Sakou T., Matsunaga S., Yamaguchi M. Family study of a twin with ossification of the posterior longitudinal ligament in the cervical spine. Spine (Phila Pa 1976). 1992. 17(3 Suppl):S55–6.

3.Okamoto K., Kobashi G., Washio M, et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); findings from a case-control study in Japan. J Bone Miner Metab. 2004. 22:612–7.

4.Sakou T., Matsunaga S., Koga H. Recent progress in the study of pathogenesis of ossification of the posterior longitudinal ligament. J Orthop Sci. 2000. 5:310–5.

5.Inamasu J., Guiot BH., Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006. 58:1027–39.

6.Hamanishi C., Tan A., Yamane T., Tomihara M., Fukuda K., Tanaka S. Ossification of the posterior longitudinal ligament. Autosomal recessive trait. Spine (Phila Pa 1976). 1995. 20:205–7.
7.Koga H., Hayashi K., Taketomi E, et al. Restriction fragment length polymorphism of genes of the alpha 2 (XI) collagen, bone morphogenetic protein-2, alkaline phosphatase, and tumor necrosis factor-alpha among patients with ossification of posterior longitudinal ligament and controls from the Japanese population. Spine (Phila Pa 1976). 1996. 21:469–73.
8.Inoue I., Ikeda R., Tsukahara S. Current topics in pharmacological research on bone metabolism: Promyelotic leukemia zinc finger (PLZF) and tumor necrosis factor-alpha-stimulated gene 6 (TSG-6) identified by gene expression analysis play roles in the pathogenesis of ossification of the posterior longitudinal ligament. J Pharmacol Sci. 2006. 100:205–10.
9.Tahara M., Aiba A., Yamazaki M, et al. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine (Phila Pa 1976). 2005. 30:877–80.

10.Inamasu J., Guiot BH., Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006. 58:1027–39.

11.Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine (Phila Pa 1976). 1989. 14:1184–91.

12.Geng L., Yao Z., Yang H., Luo J., Han L., Lu Q. Association of CA repeat polymorphism in estrogen receptor beta gene with postmenopausal osteoporosis in Chinese. J Genet Genomics. 2007. 34:868–76.
13.Khosla S., Riggs BL., Atkinson EJ, et al. Relationship of estrogen receptor genotypes to bone mineral density and to rates of bone loss in men. J Clin Endocrinol Metab. 2004. 89:1808–16.

14.Lau HH., Ho AY., Luk KD., Kung AW. Estrogen receptor beta gene polymorphisms are associated with higher bone mineral density in premenopausal, but not postmenopausal southern Chinese women. Bone. 2002. 31:276–81.
15.Morishima A., Grumbach MM., Simpson ER., Fisher C., Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995. 80:3689–98.

16.Gennari L., Merlotti D., De Paola V, et al. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review. Am J Epidemiol. 2005. 161:307–20.

17.Smith EP., Boyd J., Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994. 331:1056–61.

18.Ogata N., Koshizuka Y., Miura T, et al. Association of bone metabolism regulatory factor gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the spine and its severity. Spine (Phila Pa 1976). 2002. 27:1765–71.

19.Wang PN., Chen SS., Liu HC., Fuh JL., Kuo BI., Wang SJ. Ossification of the posterior longitudinal ligament of the spine. A case-control risk factor study. Spine (Phila Pa 1976). 1999. 24:142–4.
20.Mosselman S., Polman J., Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996. 392:49–53.
21.Bord S., Horner A., Beavan S., Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001. 86:2309–14.
22.Sims NA., Clé ment-Lacroix P., Minet D, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003. 111:1319–27.

23.Spelsberg TC., Subramaniam M., Riggs BL., Khosla S. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol. 1999. 13:819–28.

24.Pensler JM., Radosevich JA., Higbee R., Langman CB. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res. 1990. 5:797–802.

25.Lurie G., Wilkens LR., Thompson PJ, et al. Genetic polymorphisms in the estrogen receptor beta (ESR2) gene and the risk of epithelial ovarian carcinoma. Cancer Causes Control. 2009. 20:47–55.

26.Ashworth JJ., Smyth JV., Pendleton N, et al. Polymorphisms spanning the 0N exon and promoter of the estrogen receptor-beta (ERbeta) gene ESR2 are associated with venous ulceration. Clin Genet. 2008. 73:55–61.
27.Rexrode KM., Ridker PM., Hegener HH., Buring JE., Manson JE., Zee RY. Polymorphisms and haplotypes of the estrogen receptor-beta gene (ESR2) and cardiovascular disease in men and women. Clin Chem. 2007. 53:1749–56.
28.Maruyama A., Nakayama T., Sato N., Mizutani Y., Furuya K., Yamamoto T. Association study using single nucleotide polymorphisms in the estrogen receptor beta (ESR2) gene for preeclampsia. Hypertens Res. 2004. 27:903–9.
Go to : 
Table 1.
Characteristics of Study Subjects (ESR2)
Table 2.
Case-Control Association Study of ESR 2 gene in Patients with OPLL




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download