Abstract
Objectives
To evaluate the current prescription patterns of non-steroidal antiinflammatory drugs (NSAIDs) and gastrointestinal (GI) risk assessment in patients with lumbar spine disease.
Summary of Literature Review
NSAIDs are commonly prescribed medications for lumbar spine disease patients. Since the rate of GI complication varies for each patient, identification of individual GI risks is a prerequisite to prevent such a complication. There are few reports about the GI risks in patients with lumbar spine disease who take NSAIDs.
Materials and Methods
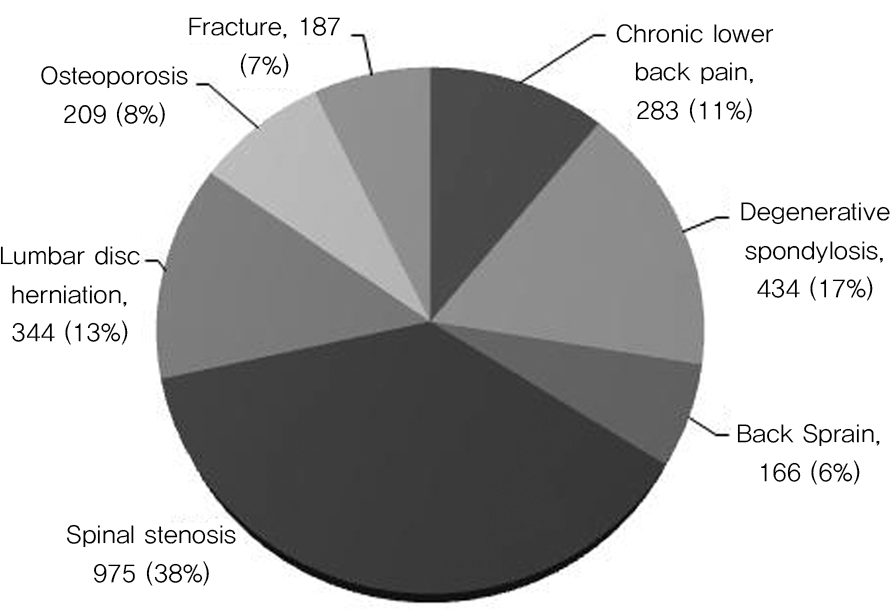
2264 patients with lumbar degenerative spondylopathy who were taking NSAIDs were enrolled from May 2010 to September 2010. The Standardized Calculator of Risk for Event (SCORE) was used to measure patients’ GI risk factors. NSAID prescription patterns and GI protective agents were also investigated.
Results
Being aged over 65 (1098 patients; 48.5%) and the presence of GI side-effects from NSAIDs (896 patients;,39.6%) were the most common risk factors. 31.9% and 5.8% percent of patients belonged to each of the high risk and the very high risk groups in GI risk factor analysis. The total prescription rate of gastroprotectants was 91.7% for all patients. However, the prescription rate of selective COX-2 inhibitors in the high risk group was low, and in 54.8% of patients who took COX-2 inhibitors there was GI discomfort.
Go to : 
REFERENCES
1. van Tulder MW, Scholten RJ, Koes BW, Deyo RA. Nonsteroidal antiinflammatory drugs for low back pain: a systematic review within the framework of The Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2000; 25:2501–13.
2. Chung JY, Lee JJ, Seo HY, Shon SJ, Chung EK. Effect of Tramadol/Acetaminophen Combination Drug in Acute Pain After Spinal Surgery. J Korean Soc Spine Surg. 2007; 14:137–43.

3. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal antiinflammatory drugs. Lancet. 1994; 343:769–72.
5. Singh G. Arthritis, Rheumatism and Aging Medical Information System Post-Marketing Surveillance Program. J Rheumatol. 2001; 28:1174–9.
6. Schlansky B, Hwang JH. Prevention of nonsteroidal antiinflammatory drug-induced gastropathy. J Gastroenterol. 2009; 44(19 Suppl):44–52.

7. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal antiinflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991; 91:213–22.

8. Fries JF, Murtagh KN, Bennett M, Zatarain E, Lingala B, Bruce B. The rise and decline of nonsteroidal antiinflammatory drug-associated gastropathy in rheumatoid arthritis. Arthritis Rheum. 2004; 50:2433–40.

9. Tramè r MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000; 85:169–82.
10. Herná ndez-Dí az S, Rodrí guez LA. Association between nonsteroidal antiinflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000; 160:2093–9.
11. Bull SA, Conell C, Campen DH. Relationship of clinical factors to the use of Cox-2 selective NSAIDs within an arthritis population in a large HMO. J Manag Care Pharm. 2002; 8:252–8.

12. Lazzaroni M, Bianchi Porro G. Non-steroidal antiinflammatory drug gastropathy: clinical results with H2 antagonists and proton pump inhibitors. Ital J Gastroenterol Hepatol. 1999; 31(1 Suppl):S73–8.
13. Daniel WW. Biostatistics: A foundation for analysis in the health science. 7thed.New York: John Wiley & Sons;1999.
14. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999; 340:1888–99.

15. Lapane KL, Spooner JJ, Pettitt D. The effect of nonsteroidal antiinflammatory drugs on the use of gastroprotective medication in people with arthritis. Am J Manag Care. 2001; 7:402–8.
16. Griffin MR. Epidemiology of nonsteroidal antiinflammatory drug-associated gastrointestinal injury. Am J Med. 1998; 30:23–9.

17. Logan R, Delaney B. ABC of the upper gastrointestinal tract: implications of dyspepsia for the NHS. BMJ. 2001; 323:675–7.

18. Lee SH, Han CD, Yang IH, Ha CW. Prescription Pattern of NSAIDs and the Prevalence of NSAID-induced Gastrointestinal Risk Factors of Orthopaedic Patients in Clinical Practice in Korea. J Korean Med Sci. 2011; 26:561–7.

19. Helsper CW, Smeets HM, Numans ME, et al. Trends and determinants of adequate gastroprotection in patients chronically using NSAIDs. Pharmacoepidemiology and drug safety. 2009; 18:800–6.

20. Chancellor JV, Hunsche E, de Cruz E, Sarasin FP. Economic evaluation of celecoxib, a new cyclo-oxygenase 2 specific inhibitor, in Switzerland. PharmacoEconomics. 2001; 19(1 Suppl):59–75.

21. Burke TA, Zabinski RA, Pettitt D, Maniadakis N, Maurath CJ, Goldstein JL. A framework for evaluating the clinical consequences of initial therapy with NSAIDs, NSAIDs plus gastroprotective agents, or celecoxib in the treatment of arthritis. PharmacoEconomics. 2001; 19(1 Suppl):33–47.

22. Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999; 282:1929–33.

23. Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal antiinflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995; 123:241–9.
Go to : 
Figures and Tables%
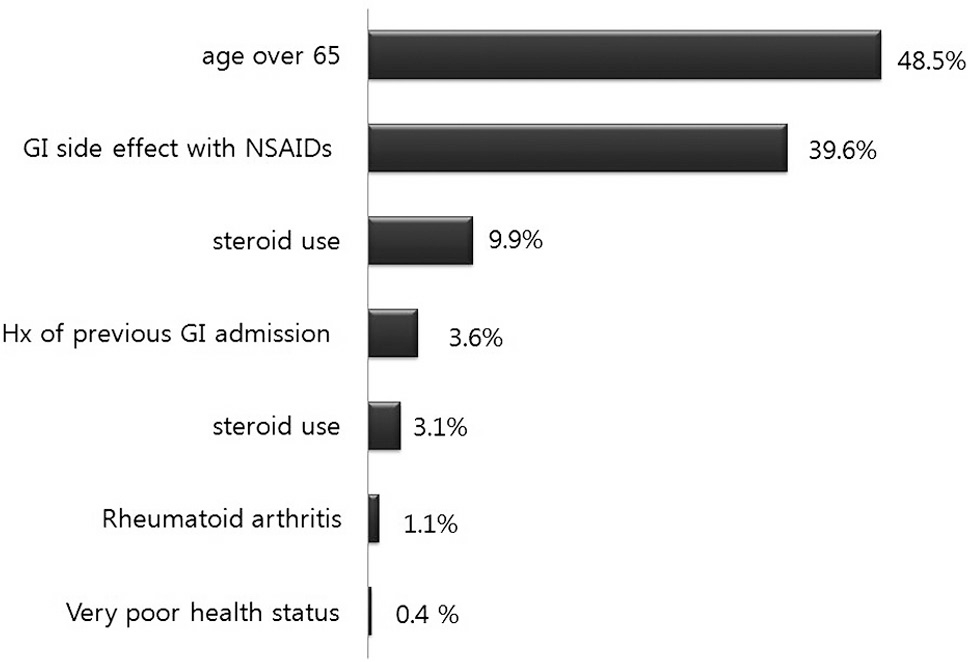 | Fig. 2.Percentage of patients with GI risk factors11) GI : gastrointestinal, NSAIDs : non-steroidal antiinflammatory drugs Hx : history |
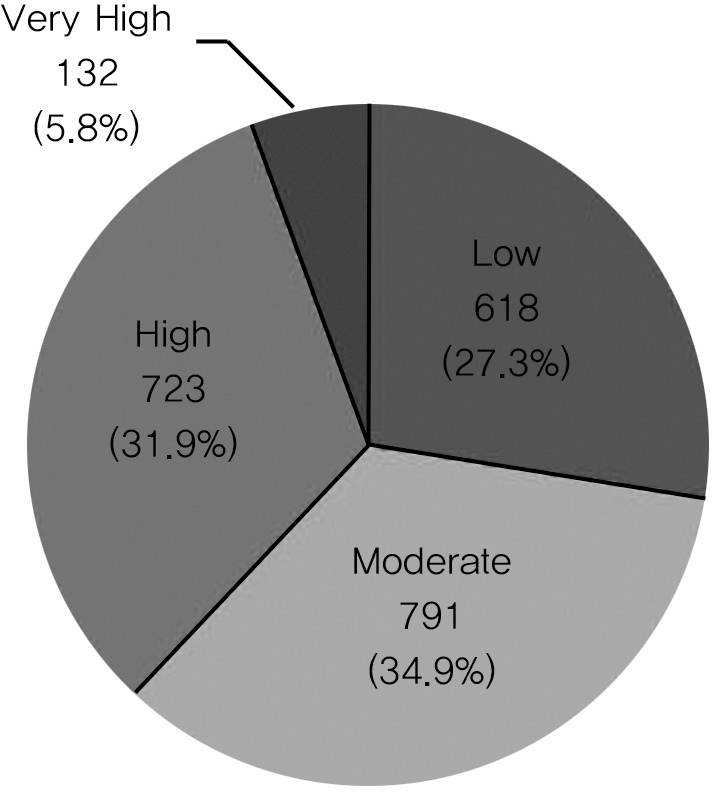 | Fig. 3.Distribution of GI risk group according to SCORE system10) is shown. The ‘very high(risk 4)’ and ‘high(risk 3)’ groups were classified as the high GI risk group. Risk 4 (very high) : more than 20 points Risk 3 (high) : 16-20 points Risk 2 (moderate) : 11-15 points Risk 1 (low) : 10 or less points |
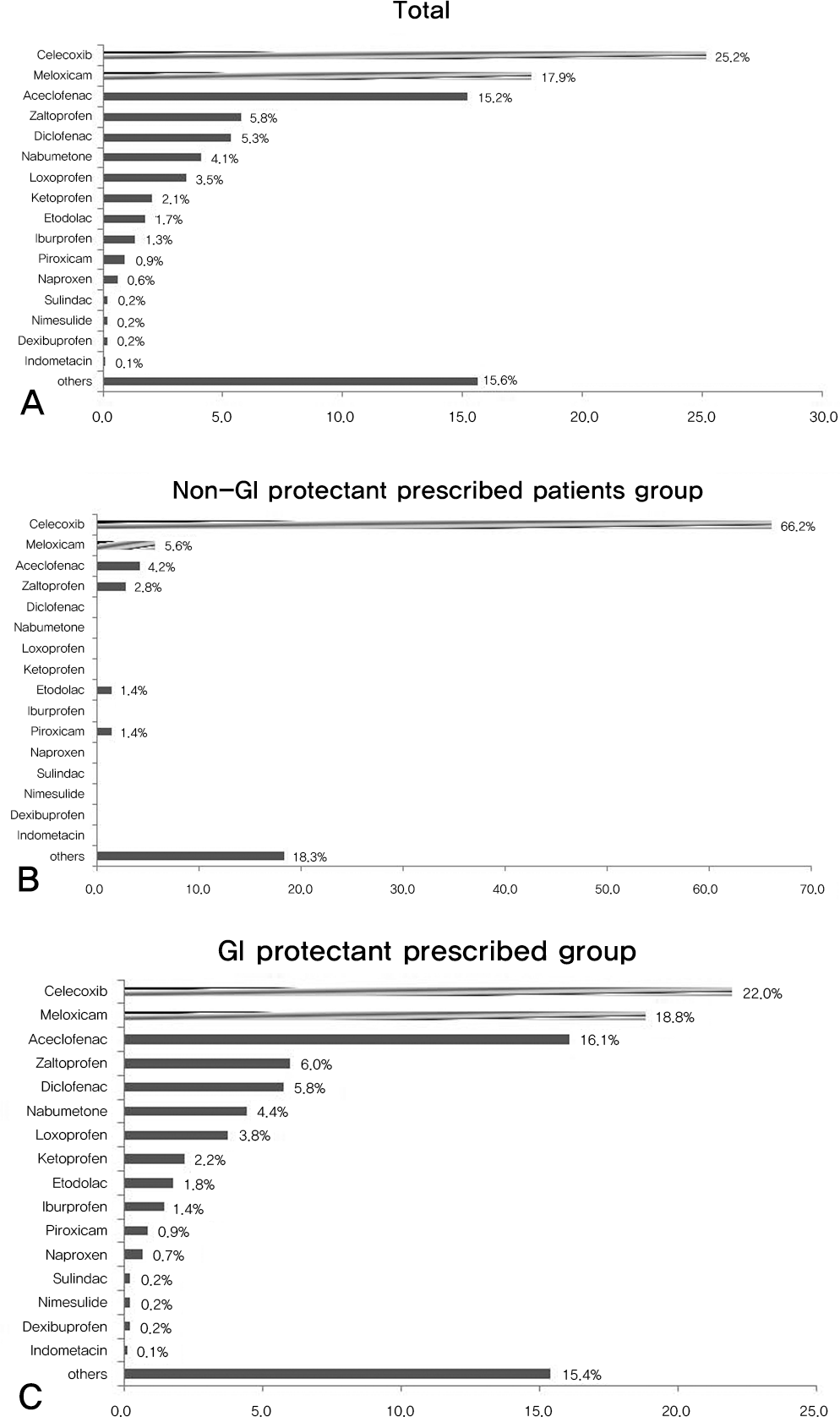 | Fig. 4.(A) Total Prescription rate of COX-2 inhibitors and other NSAIDs in high risk group are shown. The prescription rate of selective COX-2 inhibitor was 43.1% in total. (B) Prescription rate of COX-2 inhibitors and other NSAIDs in non-GI protective agent prescribed high risk patients group are shown. The prescription rate of selective COX-2 inhibitor was 71.8% in the non-GI protectant prescribed group. (C) Prescription rate of COX-2 inhibitors and other NSAIDs in GI protective agent prescribed high risk patients group are shown. The prescription rate of selective COX-2 inhibitor was 40.8% in the GI protectant prescribed group. : selective Cyclo-oxygenase type 2 inhibitor : Other Non-steroidal antiinflammatory drugs |
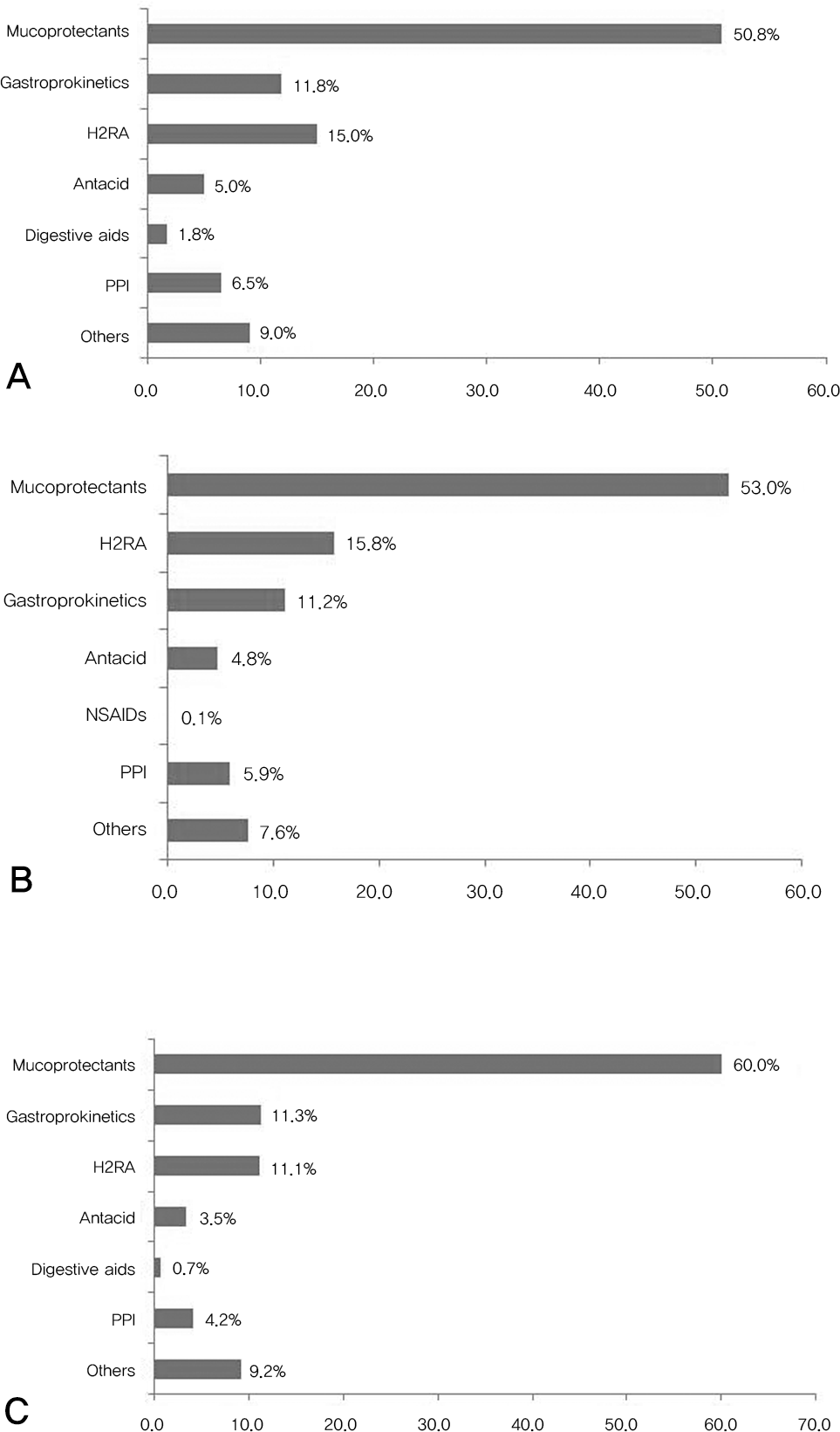 | Fig. 5.(A) Prescription Pattern of the gastroprotectives in the GI high risk group is shown. The mucoprotectants and gastroprokinetics were the most prescribed GI protective agent in order. (B) Prescription Pattern of the gastroprotectives in the GI symptomatic patients group is shown. In GI symptomatic group, the mucoprotectants and H2RA were the most prescribed. (C) Prescription Pattern of the gastroprotectives in the GI asymptomatic patients group is shown. In GI asymptomatic group, the mucoprotectants and gastroprokinetics were the most prescribed. H2RA: histamine type 2 receptor antagonist NSAIDs:non-steroidal antiinflammatory drugs PPI:proton pump inhibitor |
Table. 1.
NSAIDs GI risk SCORE Card




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download