Abstract
Study Design
This is an in-vitro experiment using rabbit intervertebral disc (IVD) cells and growth factors.
Objectives
We wanted to determine the effect of types I,and II atelocollagen and growth factor gene therapy for matrix regeneration of rabbit IVD cells.
Summary of the Literature Review
Adenovirus-medicated growth factor gene therapy is efficient for matrix regeneration of the IVD. Atellocollagen has provided a favorable environment for matrix synthesis. However, a combined approach using gene and cell therapy in an atelocollagen scaffold has not yet been attempted.
Materials and Methods
Rabbit IVD cells were transduced with Ad/TGF-β1 and Ad/BMP-2. The cells were then implanted to the atelocollagen scaffold. The [methyl-3 H]thymidine incorporation for DNA synthesis and the [35 S]sulfur incorporation for proteoglycan synthesis were measured. RT-PCR was performed for assessing the aggrecan, collagen type I, collagen type II and osteocalcin mRNA expressions.
Results
The rabbit IVD cells with Ad/TGF-β1 and that were cultured in type I atelocollagen showed a 130% increase in new proteoglycan synthesis, while the rabbit IVD cells with Ad/TGF-β1 and that were cultured in type II atelocollagen showed a 180% increase in new proteoglycan synthesis (p<0.05). The rabbit IVD cells with Ad/BMP-2 and that were cultured in type I atelocollagen showed a 70% increase in new proteoglycan synthesis, while the rabbit IVD cells with Ad/BMP-2 and that were cultured in type II atelocollagen showed a 95% increase (p<0.05). Rabbit IVD cells with Ad/TGF-β1 and Ad/BMP-2 and that were cultured in type I and II atelocollagen demonstrated increased collagen type I and II mRNA expressions without an osteocalcin mRNA expression (p<0.05).
Go to : 
REFERENCES
1.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006. 88:21–24.

2.Gibson JN., Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev. 2007. 2:CD001350.

3.David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine. 2007. 32:661–66.
4.Miyamoto K., Masuda K., Kim JG, et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006. 6:692–703.

5.Kim DJ., Moon SH., Kim H, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003. 28:2679–84.

6.Leung VY., Chan D., Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006. 15:406–13.

7.Sakai D., Mochida J., Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005. 30:2379–87.
8.Nishida K., Kang JD., Suh JK., Robbins PD., Evans CH., Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine. 1998. 23:2437–42.
9.Moon SH., Gilbertson LG., Nishida K, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine. 2000. 25:2573–79.
10.Chang G., Kim HJ., Kaplan D., Vunjak-Novakovic G., Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007. 16:1848–57.

11.Chen WH., Lo WC., Lee JJ, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006. 209:744–54.
12.O'Halloran DM., Pandit AS. Tissue-Engineering Approach to Regenerating the Intervertebral Disc. Tissue Eng. 2007. 13:1927–54.
13.Wu D., Razzano P., Grande DA. Gene therapy and tissue engineering in repair of the musculoskeletal system. J Cell Biochem. 2003. 88:467–81.

14.Lipson SJ., Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981. 6:194–210.

15.Lipson SJ., Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981. 24:12–21.
16.Butler D., Trafimow JH., Andersson GB., McNeill TW., Huckman MS. Discs degenerate before facets. Spine. 1990. 15:111–13.

17.Nishida K., Kang JD., Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999. 24:2419–25.
18.Sato M., Asazuma T., Ishihara M, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003. 64:248–56.

19.Sakai D., Mochida J., Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006. 27:335–45.

20.Sakai D., Mochida J., Iwashina T, et al. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus-like tissue in vitro: influence on the proliferation and proteoglycan production of HNPSV-1 cells. Biomaterials. 2006. 27:346–53.

21.Maldonado BA., Oegema TR Jr. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992. 10:677–90.

22.Moon SH., Nishida K., Gilbertson LG, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine. 2008. 33:1850–55.

23.Wallach CJ., Kim JS., Sobajima S, et al. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006. 6:107–12.

Go to : 
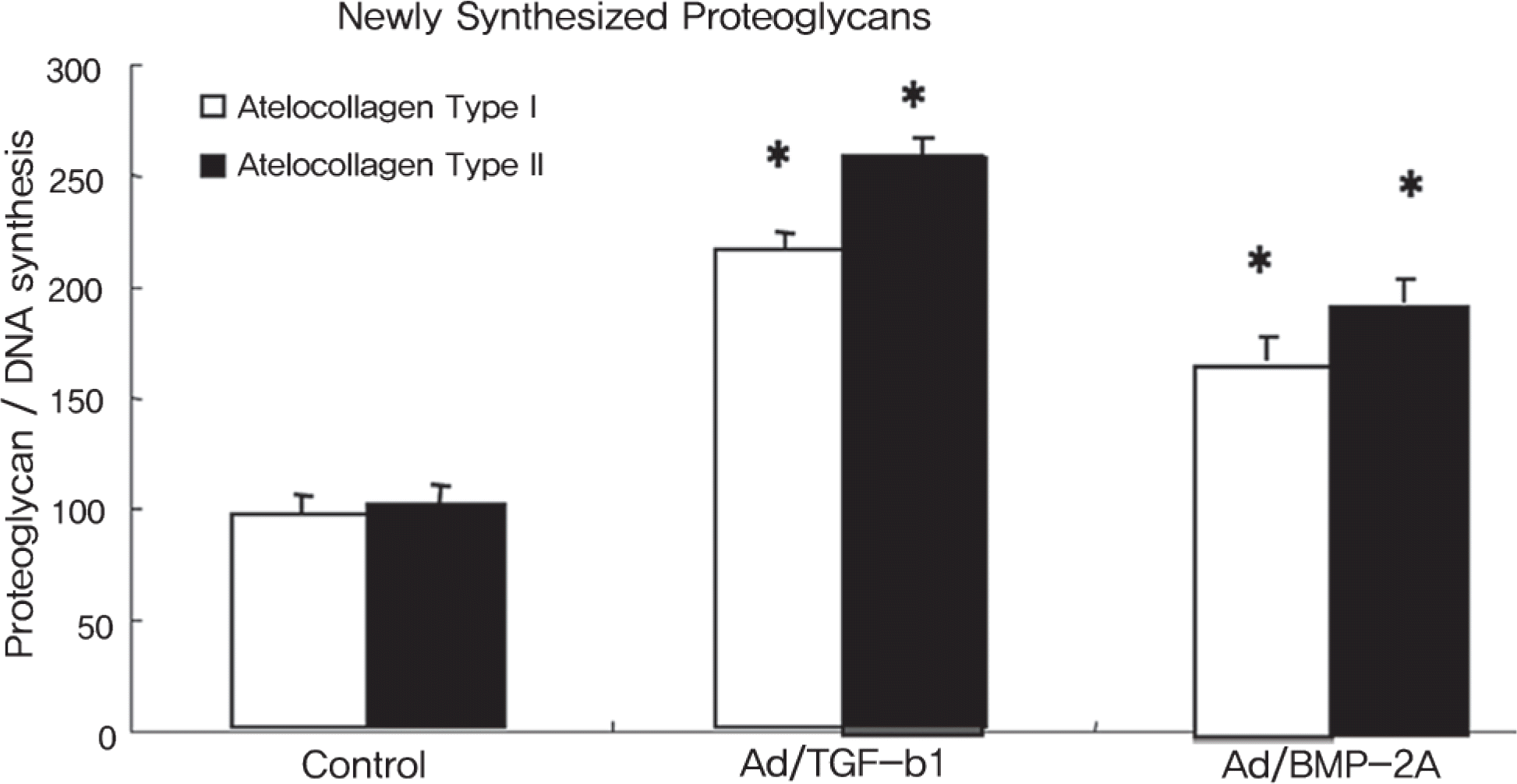 | Fig. 1.Proteoglycan synthesis([S35] incorporation) normalized by DNA synthesis([H3] incorporation) in rabbit nucleus pulposus cells seeded on atelocollagen type I and II scaffolds for culture period of 1 week. Ad/TGF-b1, Ad/BMP-2 (75MOI) were administered. Control is only cell seeded scaffold without virus infection. (∗ P<0.05) |
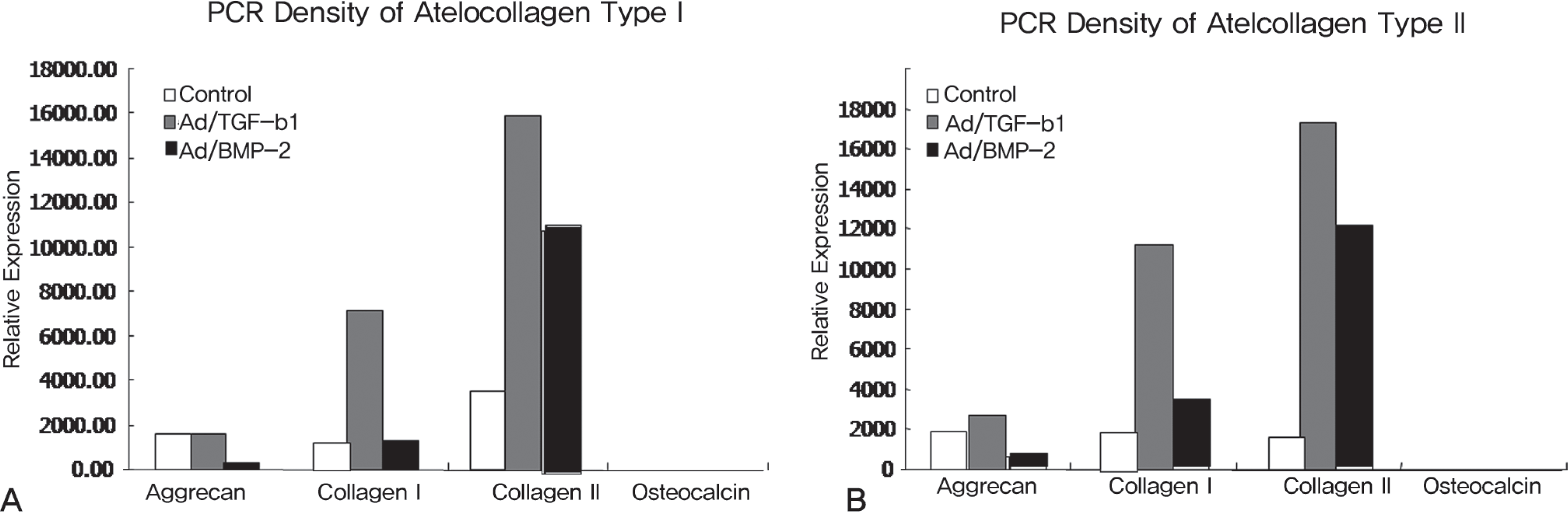 | Fig. 2.Densitometry of RT-PCR products by beta-actin expression. (A) mRNA expression of aggrecan, collagen I, II, osteocalcin for 1 week culture in atelocollagen type I matrix and (B) atelocollagen type II. |
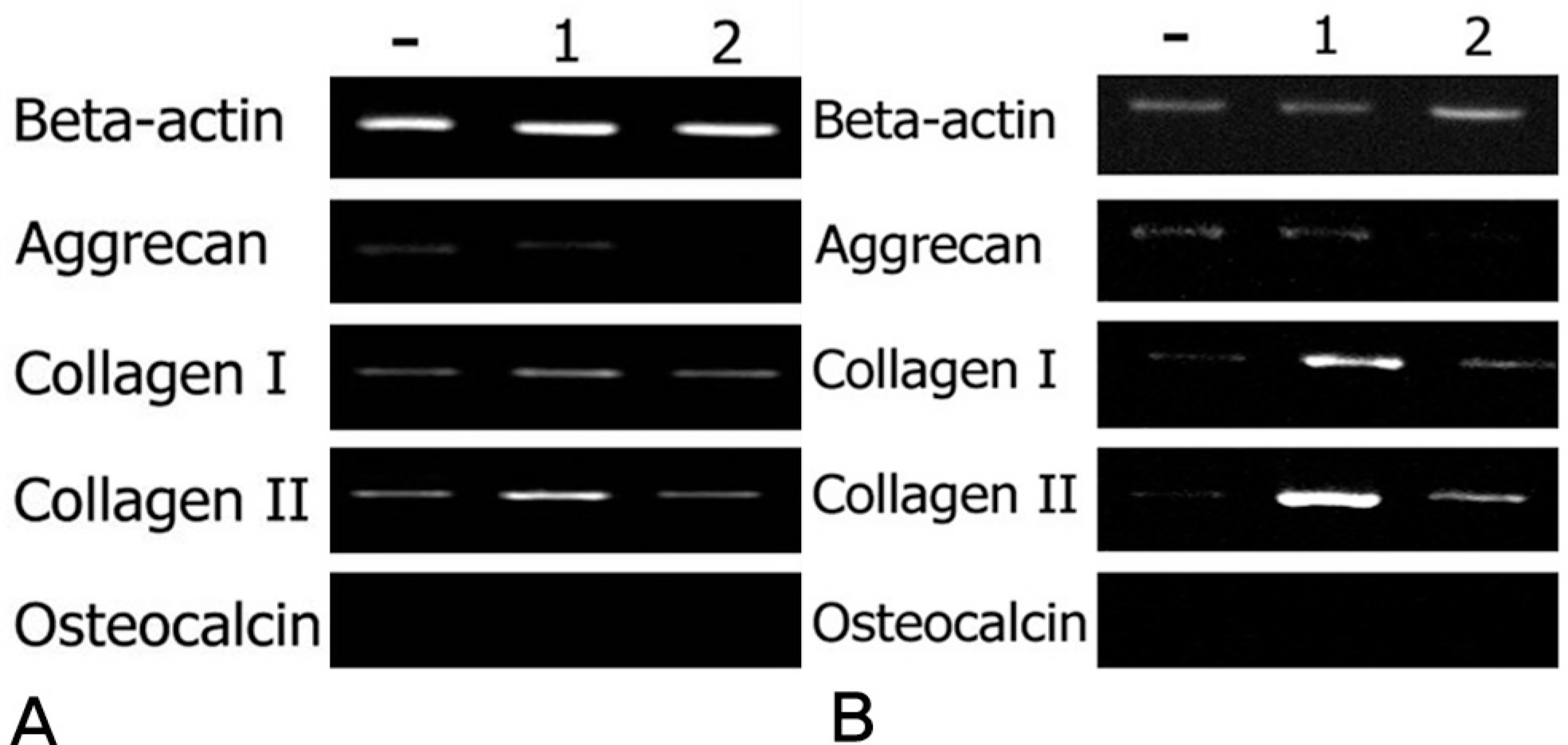 | Fig. 3. (A)RT-PCR of beta-actin, aggrecan, collagen type I, collagen type II, and osteocalcin on atelocollagen type I matrix for culture period of 1 week, (B) atelocollagen type II.- : Control (Only cell), 1 : Ad/TGF-β1, 2 : Ad/BMP-2t |
 | Fig. 4.Scanning electron microscopy of the surfaces of atelocollagen type I matrix for 1 week culture (A) and atelocollagen type II (B). Note that the cells had newly synthesized extracellular matrix (single arrow). Magnification is ×500 (A-1, B-1), ×1,000 (A-2, B-2), ×3,000 (A-3, B-3). |
Table 1.
Sequences of primers used for PCR amplification of cDNA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download