Abstract
Objectives
Increasing use of medical devices outside of healthcare facilities inevitably requires connectivity and interoperability between medical devices and healthcare information systems. To this end, standards have been developed and used to provide interoperability between personal health devices (PHDs) and external systems. ISO/IEEE 11073 standards and IHE PCD-01 standard messages have been used the most in the exchange of observation data of health devices. Recently, transmitting observation data using the HL7 FHIR standard has been devised in the name of DoF (Devices on FHIR) and adopted very fast. We compare and analyze these standards and suggest that which standard will work best at the different environments of device usage.
Methods
We generated each message/resource of the three standards for observed vital signs from blood pressure monitor and thermometer. Then, the size, the contents, and the exchange processes of these messages are compared and analyzed.
Results
ISO/IEEE 11073 standard message has the smallest data size, but it has no ability to contain the key information, patient information. On the other hand, PCD-01 messages and FHIR standards have the fields for patient information. HL7 DoF standards provide reusing of information unit known as resource, and it is relatively easy to parse DoF messages since it uses widely known XML and JSON.
Conclusions
ISO/IEEE 11073 standards are suitable for devices having very small computing power. IHE PCD-01 and HL7 DoF messages can be used for the devices that need to be connected to hospital information systems that require patient information. When information reuse is frequent, DoF is advantageous over PCD-01.
Go to : 
As the typical human life span and the importance of continuous healthcare have increased, personal health devices are being developed that allow individuals to manage their own health without going to hospitals. Vital signs observed by personal health devices are important information used to diagnose individuals' health condition. Vital signs are frequently transmitted from the client/agent to the server/manager for the purpose of storing them as personal health records (PHRs) [1], so transmission standards are being developed to provide interoperability between devices.
The ISO/IEEE 11073 standard groups are developing various standards [2] to ensure interoperability of data exchange [3] between personal health devices and external systems. The ISO/IEEE 11073 standards family defines the procedures and messages for exchanging data between a device and an external system for each personal health device. They exchange data encoded with medical device encoding rules (MDERs) [45] on a personal area network, such as USB, Serial, Bluetooth, and ZigBee.
Integrating the Healthcare Enterprise (IHE) [6] is developing profiles for sharing information in a standardized way in healthcare information systems. In particular, IHE published the IHE Patient Care Device (PCD) Device Observation Consumer (DEC) profile [7] for the transmission of observation data recorded by medical devices. The DEC profile defines PCD-01 transactions for exchanging observation data, and PCD-01 transactions are typically transported according to the simple-object access protocol (SOAP) [8] or minimal lowerlayer protocol (MLLP) [9]. Note that messages used in PCD-01 transactions are derived from the HL7 v2.6 message standard.
The HL7 Fast Healthcare Interoperability Resources (FHIR) [10] standard defines collections of health information models as resources that can be handled through RESTful web services [11]. Devices on FHIR (DoF) makes it possible to exchange observation data between personal health devices and hospital information systems by using Device, Patient, and Observation resources.
However, despite those various standards for exchanging observation data between personal health devices and external systems, it is not clear which standard will work best for the various environments of device usage.
In this paper, we compare and analyze the messages/resources, message contents, and processing data of the main standards (ISO/IEEE 11073, PCD-01 message, and DoF) for observation data transmission from health devices to external systems, and consider how to apply the most appropriate standard under certain circumstances.
Go to : 
To compare the three standards, we build standard messages/resources by applying each standard to observation data measured by a blood pressure monitor and a thermometer.
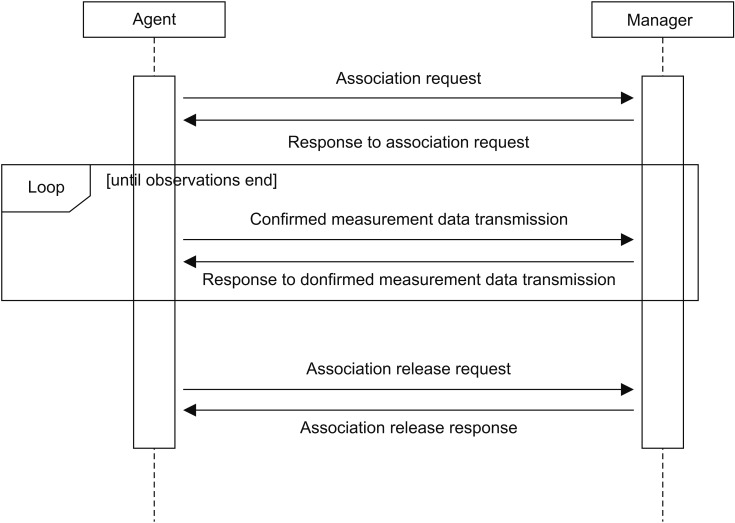
To build ISO/IEEE 11073 standard messages, we applied ISO/IEEE 11073-10407 [12] device specialization to observation data of a blood pressure monitor and ISO/IEEE 11073-10408 [13] device specialization to observation data of a thermometer. The generated messages were exchanged between a personal health device (i.e., agent) and an external system (i.e., manager) through Association, Data Reporting, and Association Release states [14] according to the standard configuration of each ISO/IEEE 11073-104zz device specialization (Figure 1).
ISO/IEEE 11073 standard messages are encoded in MDER so that they can be represented as a hexadecimal byte stream.
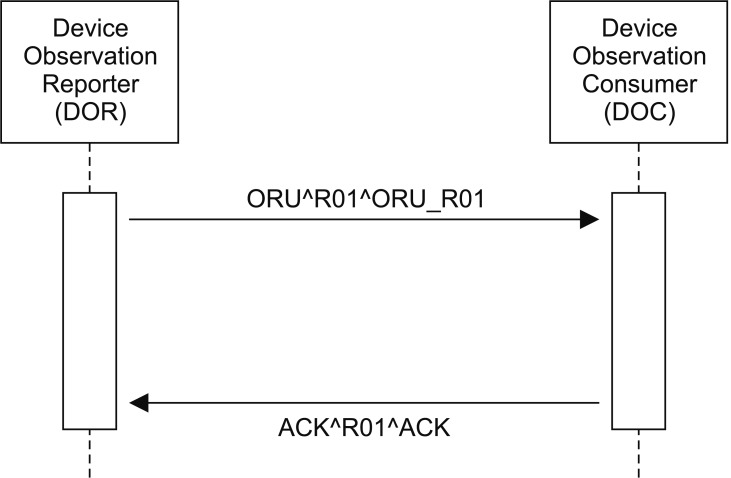
IHE PCD-01 uses the ORU^R01^ORU_R01 request message type and the ACK^R01^ACK response message type for each information exchange process or transaction [1516] (Figure 2).
An IHE PCD-01 message is derived from the HL7 v2.6 message standard with some constraints added, and it is represented as Unicode.
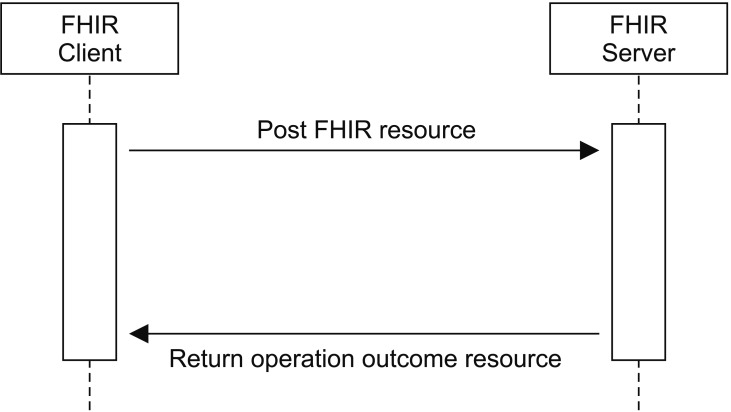
The DoF resources used to transmit observation data from personal health devices to external systems in this work were Patient, Device and Observation. In addition, we used the Bundle resource to contain a collection of resources and transmit them at one time. As in other RESTful services, an FHIR client posts resources to an FHIR server, and the server returns a result with the URL address of the created resources (Figure 3).
HL7 FHIR resources can be encoded in XML, JSON, and Turtle, but we chose JSON, because it is widely used and has relatively small overall data size.
The generated standard messages/resources were compared and analyzed as follows. First, we compared the sizes of the messages/resources to determine which standard is better for data efficiency. Second, we analyzed the message contents and information that each standard massages/resources can include. Finally, we compared the data processing of each standard, in terms of the procedure for message exchange, the difficulty of data parsing, and usability.
Go to : 
If a personal health device and an external system are associated according to the predefined standard configuration of each ISO/IEEE 11073-104zz device specialization, most of the data in ISO/IEEE 11073 messages are fixed except observation result, time, system-ID, etc., and each of those variables also have a fixed length. Therefore, the overall size of a message is fixed too. Note that this characteristic is only valid when devices are associated using standard configuration.
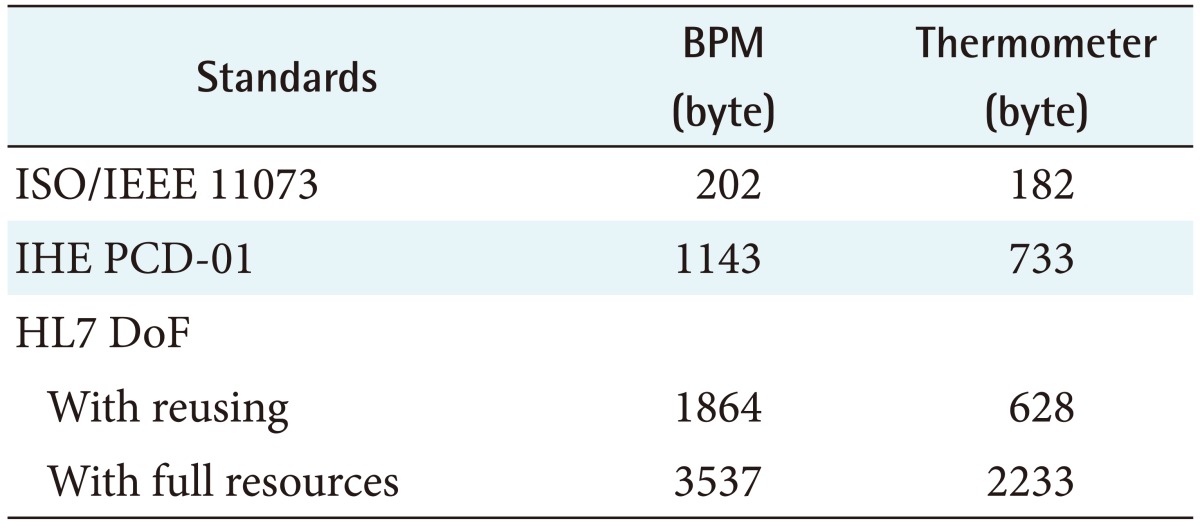
The sizes of the entire ISO IEEE 11073 messages used to exchange blood pressure monitor and thermometer's observation data were 202 and 182 bytes, respectively. Of these, the total size of the messages exchanged in the association state was 102 bytes for both blood pressure monitors and thermometers, and in the release state, the size was 12 bytes for both types of devices. The total sizes of the messages used in the data reporting state were 88 bytes for blood pressure monitors and 68 bytes for thermometers.
IHE PCD-01 messages are bigger than ISO/IEEE 11073 standard messages because they uses Unicode to provide some human readability. The sum of IHE PCD-01 request and response message sizes was 1143 bytes for a blood pressure monitor reading and 733 bytes for a thermometer reading. This result shows that the IHE PCD-01 message size was about 5.66 times larger for a blood pressure monitor reading in comparison with the ISO/IEEE 11073 standard message size and about 4.03 times larger for a thermometer reading.
For the same reason, to provide human readability, resources in DoF also have relatively large data size. DoF exchanges a total of 3237 bytes of data for a blood pressure monitor reading and a total of 1864 bytes of data for a thermometer reading. These values are about 16.02 times and 10.24 times bigger than the ISO/IEEE 11073 standard message sizes, respectively.
However, if the Device resource and the Patient resource are already posted on the FHIR server, the FHIR standard allows an FHIR client to transmit all information by simply posting Observation resources with the URL addresses of those posted resources on the FHIR server [17]. In this case, the total size of the exchanged for a blood pressure monitor reading is reduced to 2233 bytes. In particular, for thermometer data, because there is only one type of Observation, the Bundle resource is not used. As a result, the total data size exchanged is reduced to 628 bytes, which is smaller than the PCD-01 message size.
Therefore, if there is a large amount of patient information or device information, DoF may be advantageous when resources are reused.
Table 1 shows the data size comparison results of the three standards. In general, human readability and data-size efficiency of standards are inversely related.
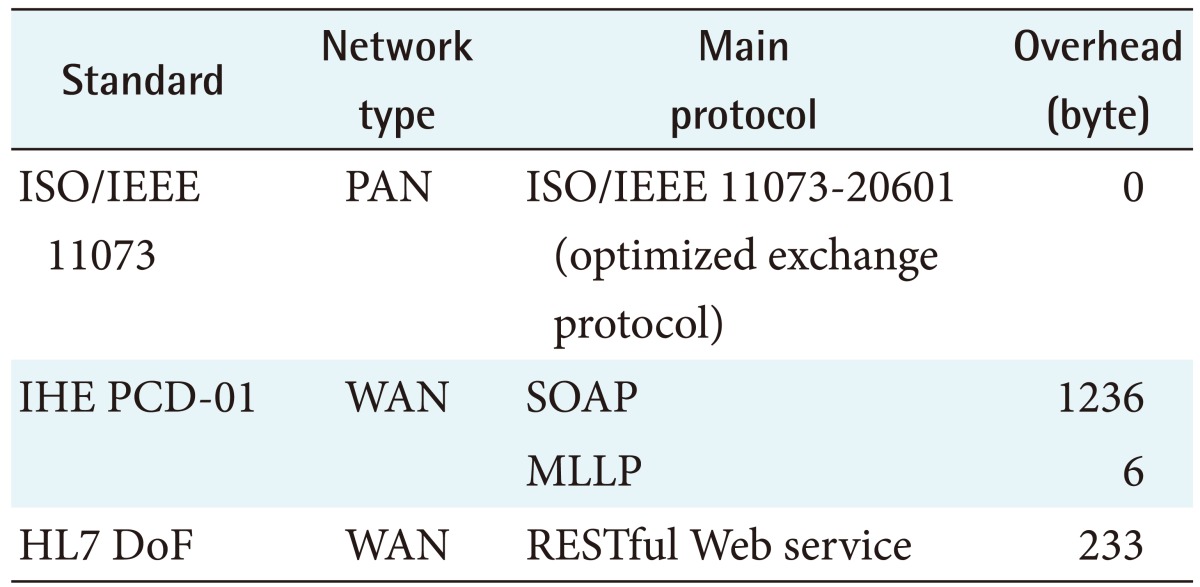
The three standards use different protocols to communicate observation data. Table 2 shows the major protocols and the overheads for the three standards.
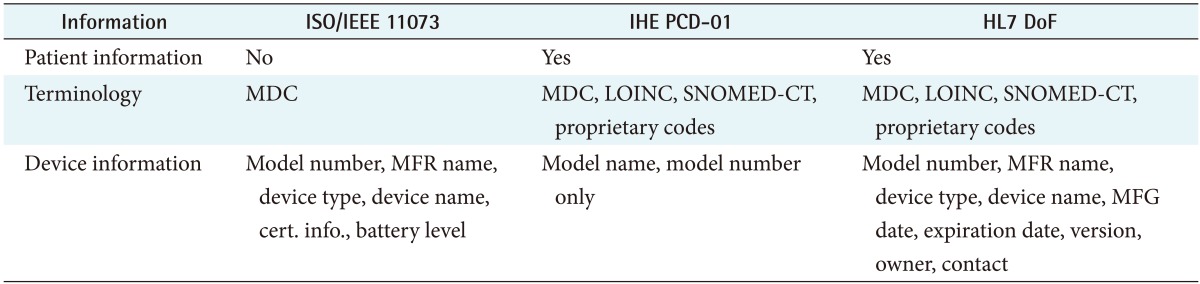
In this paper, we assume that the categories of information used to transmit measured data from health devices to external systems are Device, Observation, and Patient.
Device information consists of the type, manufacturer, model name, serial number, manufacturing date, and so forth. Observation information comprises the type of observation, the value observed by a health device, the unit of an observation, and the observation time. Finally, a Patient information includes personal information, such as a patient's name, birthdate, administrative gender, and contacts [18].
The ISO/IEEE 11073 standard provides the capability to exchange observation information, but it cannot contain patient information. If an external system wants to obtain detailed information about a personal health device, it requires an additional message exchange step not covered in this paper.
An IHE PCD-01 message can represent observation information and patient information, but only the model name or model number can be included in the health device information.
In HL7 DoF, all information exchange units are defined as resources, such as Patient, Device, and Observation, and it can provide the most detailed information among the standards covered in this paper. Furthermore, with HL7 DoF, system developers can extend all resources to provide additional information, and resources have text elements to provide descriptive information.
Table 3 compares the kind of information contained in ISO/IEEE 11073, IHE PCD-01, and HL7 DoF messages.
In summary, ISO/IEEE 11073 messages cannot contain patient information and IHE PCD-01 messages have limited device information representation, but HL7 DoF messages can contain all three major categories of information. In addition, HL7 DoF can represent additional information by extending resources, so HL7 DoF has the widest information coverage.
In the ISO/IEEE 11073 standard, to obtain measurement results from a personal health device, at least Association, Data Reporting, and Association Release steps are required. If a device and an external system are not connected by using the standard configuration, it is troublesome because more message exchanging steps are required, including the configuration information exchange step. Also, knowledge based on information is assumed to be already known by the external system. For example, the values observed by blood pressure monitors are systolic and diastolic pressures as well as MAP and pulse. An external system must know the location and order in which these values appear in a standard message. If not, a personal health device and an external system should connect using an extended configuration and exchange information about the capabilities of both sides. Then they can negotiate about what observations to exchange, what units to use, and so on.
To use information in an ISO/IEEE 11073 standard message, it is essential to interpret the MDER-encoded hexadecimal value. However, MEDR is not widely used, and it does not provide human readability, so it can be burdensome to system developer.
An IHE PCD-01 message contains a category of information per segment, and information is distinguished by a separator. Therefore, the IHE PCD-01 message structure is more intuitive than the ISO/IEEE 11073 standard message structure, and all data is represented by unicode; thus, it provides some human readability. These characteristics are convenient for processing information and delivering it to information users; it is also helpful for system developers and system users.
In the case of HL7 DoF, each information category corresponds to one resource, and resources are represented in the widely used XML or JSON format. Therefore, all information has hierarchical structures, and each element is represented with a markup tag similar to a natural language. Therefore, it is the most friendly for system developers and system users because web developers who are not familiar with DoF and the HL7 FHIR standard can also handle DoF resources. In addition, individual DoF resources themselves have complete information. For example, within an Observation resource, the resource locations of the subject patient and source device are provided as URLs. However, since an OBX segment of a PCD-01 message has only information about an observation, it is only possible to understand the full context with an entire message. Also, as mentioned earlier, HL7 DoF allows the client and server to reuse resources using URLs.
As a result, in terms of data processing, HL7 DoF is the best option because of its reusability for already posted information and its strong human readability. On the other hand, IHE PCD-01 and ISO/IEEE 11073 must resend all the information for every observation result transmission. In particular, the ISO/IEEE 11073 standard has the least usability because it should go through Association and Association Release processes to exchange observation results, and the information must undergo a relatively complicated interpretation process.
Go to : 
ISO/IEEE 11073, IHE PCD-01, and HL7 DoF standards are all capable of transmitting observed data from personal health devices. Based on our analysis, we suggest which standard is most suitable for various environments.
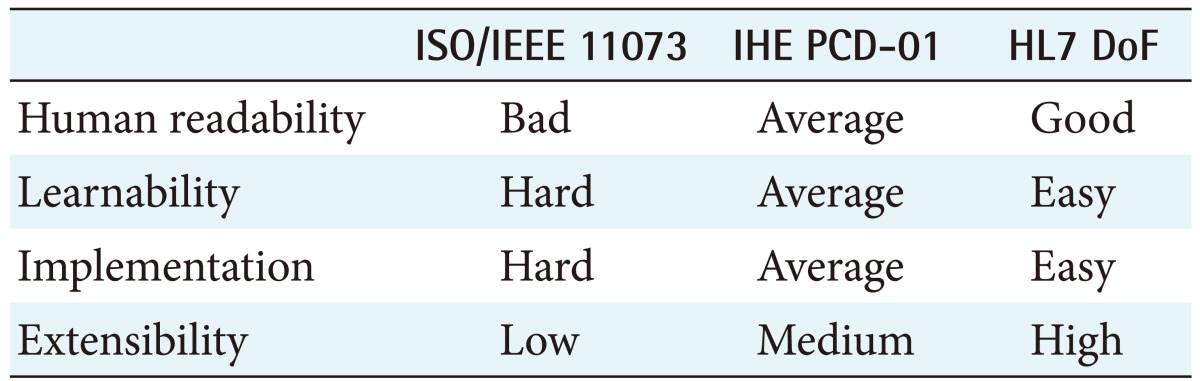
The ISO/IEEE 11073 standard is suitable for a personal health device that has relatively small computing power because its message size is the smallest [19]. Also, ISO/IEEE 11073 messages are usually transported on PAN (personal area network), so the system's communication module can be cheaper and lighter.
However, ISO/IEEE 11073 standard messages cannot contain patient information, so the receiving system or user should already know the subject patient of the observation implicitly or explicitly; otherwise, a gateway that links the patient information to the observation result is required.
IHE PCD-01 is advantageous in environments where relatively simple patient information is required and various types of transport layer are used because IHE PCD-01 messages contain patient information and typically are smaller than HL7 DoF messages. In addition, IHE PCD-01 messages can be parsed more easily than ISO/IEEE 11073 standard messages and they provide some human readability. IHE PCD-01 messages can be transported on both WAN (wide area network) and PAN, so they can be used in various communication environments.
HL7 DoF messages are the biggest and should be transported on WAN. However, they allow resources to be reused and can contain the widest variety of information. In addition, HL7 DoF is optimized for RESTful web services and represents information in widely used formats, such as XML and JSON.
For these reasons, the HL7 DoF is ideal for presenting information to end users, including online patients, after simple data processing, such as using a style sheet [20].
Table 4 shows the qualitative analysis of the ISO/IEEE 11073, IHE PCD-01 and HL7 DoF standards.
In this paper, we compared three standards (ISO/IEEE 11073, IHE PCD-01, and HL7 DoF) that are used for the transmission of observed data measured by personal health devices. Through an analysis, we identified the advantages and disadvantages of each standard, and we determined which standard is appropriate in various circumstances. Future research should implement and test these standards in a real communication environment and develop solutions for possible problems.
Go to : 
References
1. Tang PC, Ash JS, Bates DW, Overhage JM, Sands DZ. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006; 13(2):121–126. PMID: 16357345.

2. Do H, In J, Lee S. Implementation of ASN.1 converter for applying ISO/IEEE 11073 MDER. J Korean Inst Inf Technol. 2012; 10(4):19–30.
3. Institute of Electrical and Electronics Engineers. IEEE Standard Computer Dictionary: a compilation of IEEE Standard Computer Glossaries 610. New York (NY): Institute of Electrical and Electronics Engineers;1991.
4. International Organization for Standardization. Health informatics—Point-of-care medical device communication—Part 20101: Application profiles—Base standard. Geneva, Switzerland: International Organization for Standardization;2004. ISO/IEEE 11073-20101:2004.
5. Yuksel M, Dogac A. Interoperability of medical device information and the clinical applications: an HL7 RMIM based on the ISO/IEEE 11073 DIM. IEEE Trans Inf Technol Biomed. 2011; 15(4):557–566. PMID: 21558061.

6. Integrating the Healthcare Enterprise [Internet]. Oak Brook (IL): IHE International;c2016. cited at 2018 Jan 10. Available from: http://www.ihe.net.
7. Integrating the Healthcare Enterprise. IHE Patient Care Device (PCD) technical framework (volume 1) [Internet]. Oak Brook (IL): IHE International;c2017. cited at 2018 Jan 10. Available from: https://www.ihe.net/Technical_Frameworks/#pcd.
8. W3C Recommendation. SOAP Version 1.2 Part 0: Primer (second edition) [Internet]. Cambridge (MA): World Wide Web Consortium;2007. cited at 2018 Jan 10. Available from: https://www.w3.org/TR/soap12-part0/.
9. Spronk R. Transport Specification: MLLP, Release 1. Ann Arbor (MI): Health Level Seven International;2003.
10. Health Level Seven International. FHIR Release 3 (STU) [Internet]. Ann Arbor (MI): Health Level Seven International;c2011. cited at 2018 Jan 10. Available from: https://www.hl7.org/fhir/.
11. Fielding RT. Architectural styles and the design of network- based software architectures [dissertation]. Irvine (CA): University of California;2000.
12. International Organization for Standardization. Health informatics—Personal health device communication—Part 10407: Device specialization—Blood pressure monitor. Geneva, Switzerland: International Organization for Standardization;2008. ISO/IEEE 11073-10407:2008.
13. International Organization for Standardization. Health informatics—Personal health device communication—Part 10408: Device specialization—Thermometer. Geneva, Switzerland: International Organization for Standardization;2008. ISO/IEEE 11073-10408:2008.
14. International Organization for Standardization. Health informatics—Personal health device communication—Part 20601: Application profile—Optimized exchange protocol. Geneva, Switzerland: International Organization for Standardization;2014. ISO/IEEE 11073-20601:2014.
15. Health Level Seven International. HL7 Messaging Standard Version 2.6 [Internet]. Ann Arbor (MI): Health Level Seven International;2007. cited at 2018 Jan 10. Available from: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=145.
16. Rhoads JG, Cooper T, Fuchs K, Schluter P, Zambuto RP. Medical device interoperability and the Integrating the Healthcare Enterprise (IHE) initiative. Biomed Instrum Technol. 2010; (Suppl):21–27.
17. Health Level Seven International. HL7 Version 3 Specification: hData Record Format, Release 1 [Internet]. Ann Arbor (MI): Health Level Seven International;2014. cited at 2018 Jan 10. Available from: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=261.
18. Dolin RH, Alschuler L, Behlen F, Biron PV, Boyer S, Essin D, et al. HL7 document patient record architecture: an XML document architecture based on a shared information model. Proc AMIA Symp. 1999; 52–56. PMID: 10566319.
19. Clarke M, Bogia D, Hassing K, Steubesand L, Chan T, Ayyagari D. Developing a standard for personal health devices based on 11073. Conf Proc IEEE Eng Med Biol Soc. 2007; 2007:6175–6177. PMID: 18003430.

20. Clark J, Pieters S, Thompson HS. Associating stylesheets with XML documents 1.0 (second edition). Cambridge (MA): World Wide Web Consortium;2010.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download