Abstract
Objectives
Long-term follow-up care after total joint arthroplasty is essential to evaluate hip and knee arthroplasty outcomes, to provide information to physicians and improve arthroplasty performance, and to improve patients' health condition. In this paper, we aim to improve the communication between arthroplasty patients and physicians and to reduce the cost of follow-up controls based on mobile application technologies and cloud computing.
Methods
We propose a mobile-based healthcare system that provides cost-effective follow-up controls for primary arthroplasty patients through questions about symptoms in the replaced joint, questionnaires (WOMAC and SF-36v2) and the radiological examination of knee or hip joint. We also perform a cost analysis for a set of 423 patients that were treated in the University Clinic for Orthopedics in Essen-Werden.
Joint arthroplasty is one of the most common procedures performed in the United States. It is estimated that the number of total hip arthroplasties (THA) will increase by 174% and that of total knee arthroplasties (TKA) will increase by 673% per year in the Unite States by 2030 [1]. Despite the increasing frequency of these procedures, continuing follow-up assessments are essential. Routine follow-up is essential to detect asymptomatic patients suffering from important issues (e.g., ‘silent’ osteolysis) and treat them accordingly to avoid deterioration of the clinical condition [234].
Many studies [56789] have investigated the frequency of follow-up assessments and the importance of supporting patients after THA or TKA. The minimum requirements suggested by the British Orthopaedic Association are radiologic assessments every 5 years for TKA, and at 12 months, 7 years postoperatively and every 3 years thereafter for THA [1011].
In this paper, we adapt the mobile-based healthcare system proposed by Bitsaki et al. [12] for offering services to arthroplasty patients who are subject to surgical procedures and need follow-up support. The proposed system uses a combination of cloud- and service-oriented computing, online services, data analysis, and mhealth applications to connect patients to their physicians. The system is able to monitor patients and promptly recognize impeding complications, resulting in the reduction of cost and time spent by doctors, patients, and patient escorts.
A number of studies have evaluated the use of telemedicine for the follow-up of arthroplasty patients. Wood et al. [13] conducted a randomized trial on arthroplasty patients comparing an electronic clinic with standard clinic visits. The reported travel costs were $20 for an outpatient clinic and $18 for an electronic clinic.
Marsh et al. [14] showed statistically significant differences between web-based and in-clinic follow-up assessment groups in the mean travel costs (Can$10.45 vs. Can$21.36). Sharareh and Schwarzkopf [9] used Skype calls for the follow-up of total joint arthroplasty patients and found a statistical significant reduction in unplanned clinic visits (14 vs. 3) and calls (40 vs. 6).
Jeong and Kim [15] developed a web-based computer-tailored education program for the promotion of self-care for THA patients. The validity of the developed program's content and design was confirmed through an expert evaluation.
Various studies have provided evidence for the usefulness of mobile applications in orthopedics. A systematic review of the literature [16] described all validated accelerometer-, magnetometer-, and photographic-based smartphone applications used as goniometers for peripheral joints and the spine. Researchers in Australia [17] more accurately measured the rotational deformity of a correction osteotomy using an application for iPhone. Peters et al. [18] used an iPhone for acetabular cup placement in THA. Hawi et al. [19] accurately estimated the femoral anteversion on cadaveric femora using the gyroscope of an iPhone. Roberts et al. [20] used a reminder letter, a reminder SMS (short message service), and access to a tablet computer in clinic.
The most interesting feature of our approach that has not been considered in other studies is its ability to support data processing by means of cloud computing technologies to help physicians dynamically adjust treatments and share patient information in a cost-effective way.
We propose a mHealth system that provides health services to both patients and physicians as described below. The patients will give the written consent, download the application, and register during the first follow-up visit.
The follow-up and the interactions of the patients, the doctors, and the system are described by the following algorithm of action:
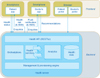
The environment that we will use to implement our services in this paper has been presented by Bitsaki et al. [12]. It consists of a frontend (iOS and Android smartphone mobile application) and a backend (Health Server) as shown in Figure 1. The publisher's permission to use the Figures 1, 2, 3, 4 was obtained. Through the application, patients securely transmit follow-up data to their doctors and have feedback on a scheduled basis.
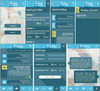
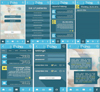
The graphical user interface of the application (Figures 3, 4) consists of screens: “Login”, “Report” (for patient), or “List of patients” (for doctor), “Reports history”, “Message”, and “Settings”.
All healthcare data (entered by doctors or patients) are stored in the Health Data component of the Health Server. Health Services and Analytics Services manage and process the healthcare data, such as patient data, insurance data, prescriptions, etc.
A cost analysis was performed to compare the proposed mHealth solution with the traditional way of supporting follow-up in terms of healthcare and travel costs per patient (per year).
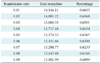
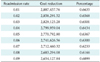
The cost estimations were based on input taken from primary hip and knee replacement surgeries performed at the University Clinic for Orthopedics in Essen-Werden per year (Table 1). For the estimation of the healthcare costs we considered that patients subscribe to the application one year after their operation and they adopt a follow-up scheme (a radiological examination and perhaps a visit to the hospital) that requires a new assessment once a year for the next 10 years. Visits can be avoided depending on the radiological assessment, the scores of the questionnaires, and the answers to the questions provided by the application as defined in the Algorithm of Action. For our analysis we assumed that the readmission rate r (percentage of patients that are recommended to visit their physician at the hospital) fluctuates within the range [0.01, 0.1]. In Table 2 we provide the standard costs and the reduced costs due to the use of the mHealth platform for the 423 patients of Table 1 for one follow-up assessment for r = 0.05. The total cost reduction with the proposed approach is €13,578.
In Table 3, we present the cost reduction for various values of r per year. Figure 5 shows the graphical representation of the cost reduction as a function of r. These results show significant cost savings. For example, for a readmission rate of 5%, the cost reduction reaches the percentage of 63.67% of the standard healthcare total cost of all hip and knee replacement patients.
To show the degree of cost reduction on a wider scale, we performed our cost analysis for the whole population of the state of North Rhine-Westphalia in Germany (17,638,098 inhabitants) [23]. For the analysis, we considered that in North Rhine-Westphalia the current rate of patients who undergo hip and knee replacement is the same as in all of Germany, which is 284/100,000 inhabitants for hip replacement and 206/100,000 for knee replacement [24]. The results are shown in Table 4.
Our approach will spur innovation across a number of industries. Mobile applications for health services will get a boost from their ability to connect to data that can be managed and analyzed by specialized services running on the proposed platform. In addition, our approach will encourage innovation in the fields of data analytics, risk models, etc. to be applied across a broad spectrum of interventions. We anticipate that our approach will accelerate societal and possibly economic change in several areas. First and foremost, patients will feel strongly empowered to self-manage their disease in cooperation with their healthcare providers. Our approach to personalized care will increase the level of education of patients and caregivers regarding ICT solutions and will strengthen the knowledge about patients' behavior related to the prevention of complications. We also expect that in the long-term overall health costs and insurance premiums will fall, improving the management of follow-up controls by reducing the number of severe episodes, hospital emergency visits, and complications.
In addition, the scientific community will be able to use the data collected through our platform for research purposes. The electronic platform could also be used after modifications for the collection of data in national arthroplasty registries.
The results of the present study depend on the fact that the follow-up intervals for arthroplasty patients show great variation internationally. Another restriction is that the readmission rate cannot be estimated accurately. Lack of data urged us to make the assumption that in North Rhine-Westphalia the current rate of patients who undergo total hip and knee arthroplasty is the same as that in all of Germany. Finally, the generalization of the results may not be appropriate in different countries with great differences in their insurance systems.
Figures and Tables
References
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007; 89(4):780–785.

2. Haddad FS, Ashby E, Konangamparambath S. Should follow-up of patients with arthroplasties be carried out by general practitioners? J Bone Joint Surg Br. 2007; 89(9):1133–1134.

3. Lavernia CJ. Cost-effectiveness of early surgical intervention in silent osteolysis. J Arthroplasty. 1998; 13(3):277–279.

4. Lindahl H, Oden A, Garellick G, Malchau H. The excess mortality due to periprosthetic femur fracture: a study from the Swedish national hip arthroplasty register. Bone. 2007; 40(5):1294–1298.

5. Hacking C, Weinrauch P, Whitehouse SL, Crawford RW, Donnelly WJ. Is there a need for routine follow-up after primary total hip arthroplasty? ANZ J Surg. 2010; 80(10):737–740.

6. Maru M, Auyeung J, Irwin L. Primary total hip replacement follow-up study. Eur J Orthop Surg Traumatol. 2005; 15(4):286–288.

7. Lieberman JR, Leger RR, Tao JC, Clohisy JC, Meneghini RM. Total hip arthroplasty surveillance: when do we see our patients postoperatively? J Arthroplasty. 2011; 26(8):1161–1164.

8. Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: results of the American Association of Hip and Knee Surgeons' member survey. J Arthroplasty. 2003; 18(8):954–962.

9. Sharareh B, Schwarzkopf R. Effectiveness of telemedical applications in postoperative follow-up after total joint arthroplasty. J Arthroplasty. 2014; 29(5):918–922.e1.

10. British Orthopaedic Association. Primary total hip replacement: a guide to good practice. London: British Orthopaedic Association;2006.
11. British Orthopaedic Association. Knee replacement: a guide to good practice. London: British Orthopaedic Association;1999.
12. Bitsaki M, Koutras C, Koutras G, Leymann F, Steimle F, Wagner S, et al. ChronicOnline: Implementing a mHealth solution for monitoring and early alerting in chronic obstructive pulmonary disease. Health Informatics J. 2016; 04. 21. [Epub]. DOI: 10.1177/1460458216641480.

13. Wood G, Naudie D, MacDonald S, McCalden R, Bourne R. An electronic clinic for arthroplasty follow-up: a pilot study. Can J Surg. 2011; 54(6):381–386.

14. Marsh J, Bryant D, MacDonald SJ, Naudie D, Remtulla A, McCalden R, et al. Are patients satisfied with a web-based followup after total joint arthroplasty? Clin Orthop Relat Res. 2014; 472(6):1972–1981.

15. Jeong YW, Kim JA. Development of computer-tailored education program for patients with total hip replacement. Healthc Inform Res. 2014; 20(4):258–265.

16. Milani P, Coccetta CA, Rabini A, Sciarra T, Massazza G, Ferriero G. Mobile smartphone applications for body position measurement in rehabilitation: a review of goniometric tools. PM R. 2014; 6(11):1038–1043.

17. Graham D, Suzuki A, Reitz C, Saxena A, Kuo J, Tetsworth K. Measurement of rotational deformity: using a smartphone application is more accurate than conventional methods. ANZ J Surg. 2013; 83(12):937–941.

18. Peters FM, Greeff R, Goldstein N, Frey CT. Improving acetabular cup orientation in total hip arthroplasty by using smartphone technology. J Arthroplasty. 2012; 27(7):1324–1330.

19. Hawi N, Kabbani AR, O'Loughlin P, Krettek C, Citak M, Liodakis E. Intraoperative measurement of femoral antetorsion using the anterior cortical angle method: a novel use for smartphones. Int J Med Robot. 2013; 9(1):29–35.

20. Roberts N, Bradley B, Williams D. Use of SMS and tablet computer improves the electronic collection of elective orthopaedic patient reported outcome measures. Ann R Coll Surg Engl. 2014; 96(5):348–351.

21. Bellamy N. Osteoarthritis: an evaluative index for clinical trials [master's thesis]. Hamilton, Canada: McMaster University;1982.
22. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I: Conceptual framework and item selection. Med Care. 1992; 30(6):473–483.
23. Official population of Nordrhein-Westfalen [Internet]. Dusseldorf: IT.NRW.com;c2015. cited at 2017 Jan 3. Available from: https://www.it.nrw.de/presse/pressemitteilungen/2015/pres_241_15.html.
24. Wengler A, Nimptsch U, Mansky T. Hip and knee replacement in Germany and the USA: analysis of individual inpatient data from German and US hospitals for the years 2005 to 2011. Dtsch Arztebl Int. 2014; 111(23-24):407–416.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download