This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS) is one of the major burdens of disease in developing countries, and the standard-of-care treatment includes prescribing antiretroviral drugs. However, antiretroviral drug resistance is inevitable due to selective pressure associated with the high mutation rate of HIV. Determining antiretroviral resistance can be done by phenotypic laboratory tests or by computer-based interpretation algorithms. Computer-based algorithms have been shown to have many advantages over laboratory tests. The ANRS (Agence Nationale de Recherches sur le SIDA) is regarded as a gold standard in interpreting HIV drug resistance using mutations in genomes. The aim of this study was to improve the prediction of the ANRS gold standard in predicting HIV drug resistance.
Methods
A genome sequence and HIV drug resistance measures were obtained from the Stanford HIV database (
http://hivdb.stanford.edu/). Feature selection was used to determine the most important mutations associated with resistance prediction. These mutations were added to the ANRS rules, and the difference in the prediction ability was measured.
Results
This study uncovered important mutations that were not associated with the original ANRS rules. On average, the ANRS algorithm was improved by 79% ± 6.6%. The positive predictive value improved by 28%, and the negative predicative value improved by 10%.
Conclusions
The study shows that there is a significant improvement in the prediction ability of ANRS gold standard.
Keywords: Medical Informatics, Health Informatics, Computational Biology, Artificial Intelligence, Clinical Informatics, Machine Learning
I. Introduction
Developing countries are characterized by poor infrastructure and limited resources. The World Health organization has indicated that with the current financing strategy, many developing countries do not meet the requirements for universal healthcare coverage. Angola spends 3.4% of their GDP on healthcare expenditure. Republic of Congo spends 2.5%; Eritrea spends 2.6%, and Kuwait spends 2.7% of their GDP on health compared to the Unites States which spends 17.9% of their GDP [
1]. Developing countries struggle under the burden of human immunodeficiency virus (HIV), tuberculosis and malaria [
2]. HIV is an incurable disease which affects the functioning of the immune system of a human over a long period of time.
There are currently almost 6.4 million people infected with HIV in South Africa, which is approximately 12.2% of the South African population [
34]. Swaziland has a HIV infection prevalence of 26%, Botswana 25%, and Lesotho 24% [
5]. The burden of HIV in Africa is understood when acknowledging the contrast with the prevalence of HIV in developed countries. France and Spain have a prevalence of 0.4%, while Netherland has a prevalence of 0.2%. It is also estimated that there are almost 500,000 patients who exhibit AIDS defining conditions in South Africa [
6].
HIV is managed by highly active antiretroviral therapy, which consists of antiretroviral (ARV) drugs from protease inhibitors, reverse transcriptase inhibitors, integrate inhibitors, fusion inhibitors, and entry inhibitors. However, the success of managing HIV with ARVs is dependent on the actual treatment, stage of the disease, drug potency, patient adherence, achievable drug concentrations, drug resistance and toxic effects of the drugs [
78]. Of these factors, drug resistance is crucial and defined as the diminished ability of antiretroviral drugs to reduce the HIV viral load adequately [
2].
HIV drug resistance is inevitable due to selective pressure facilitated by the presence of ARVs during the management of HIV, high replication errors of the virus and initial infection [
8]. Thus, the ability to easily determine drug resistance is vital in the treatment of HIV positive patients. HIV drug resistance is normally tested using phenotypic tests [
9].
Briefly, phenotypic tests work by analyzing the concentration of ARV that is required to reduce the reproduction of a laboratory grown sample of the HIV that has infected a specific patient by 50%. The ratio of this concentration over the concentration required when using the wild type (original) HIV virus is called the IC
50. The IC
50 score is compared to cutoff values obtained from the literature and is thus characterized as being either resistant to ARV drugs, susceptible to ARV drugs or intermediate resistance to ARV drugs. Although the IC
50 score is seen as the absolute measurement, laboratory based tests are relatively expensive, time consuming, and susceptible to errors, and each test detects resistance to a single drug, and thus, many tests are required to determine multiple drug resistances [
10]. With the current disease burden and lack of resources in developing countries, phenotypic tests are not viable.
Electronic computerized algorithms [
11] (a part of the medical informatics domain) may also be used to determine ARV drug resistance and have many advantages over phenotype testing. Computer-based genotype interpretation algorithms usually determine mutations in a patient's pol gene and uses this information to determine which ARV drugs the patient is resistant to. The literature has associated mutations with particular resistance profiles. These computer based tests are faster and cheaper than phenotypic tests.
One widely used computer based interpretation algorithm was built by the French ANRS (Agence Nationale de Recherches sur le SIDA; National Agency for AIDS Research) AC11 Resistance group and is called the Agence Nationale de Recherches sur le SIDA. ANRS is seen as a gold standard in interpreting HIV drug resistance using mutations in genomes. ANRS classifies ARV resistance according to three levels: susceptible, intermediate, and resistant. ‘Susceptible’ indicates that a particular ARV drug will be effective against HIV; ‘intermediate’ indicates that the ARV drug is partially effective; and if the ARV is not effective at all, it is classified ‘resistant’.
ANRS algorithm is based on a linear combination of mutations. If a particular mutation or a group of mutations are present in the genome, the algorithm returns a resistance profile applicable to that particular sequence, e.g., if the mutation A98S is present in the genome, resistance to the NVP is deduced, whereas an E138K mutation will indicate intermediate resistance to the NVP drug.
Each rule consists of a Boolean expression. For example, an ANRS rule for abacavir (version 13, July 2005) states: “If there are five or more of the following RT mutations (M41L, D67N, L74V, M184V/I, L210W, T215Y/F), report resistance to abacavir” [
12].
The ANRS system bases its interpretations almost entirely on genotype outcome studies, and the ANRS has published a large proportion of studies linking genotype to the virological outcome, including studies on genotypic predictors of response to abacavir [
13], tenofovir [
13], and didanosine [
14].
ANRS is based on an experts' understanding of the domain and available datasets as well as the published literature. Therefore, there may be discrepancies and room for improvement. The average accuracy of ANRS was 59% [
2]. Thus, the aim of this study was to use machine learning to see if there can be improvements in the effectiveness of ANRS in predicting HIV drug resistance.
II. Methods
The methods used in this paper are divided into three parts: data-processing, development of an association matrix, and the determination of the effectiveness of ANRS with the incorporation of the association matrix.
1. Data Processing
Free publically available and de-identified genotype-phenotype datasets that consisted of approximately 23,000 protease (PR) gene and 23,000 reverse transcriptase (RT) gene sequences were obtained from the Stanford HIV drug resistance database (
http://hivdb.stanford.edu/).
These datasets were fed into the ANRS algorithm, and a resistance measure was obtained for each sequence. The ANRS result was then compared to the known resistance measure obtained from a laboratory test for each sequence, and the accuracy of the ANRS algorithm was calculated.
2. Development of the Association Matrix
Machine learning is an artificial intelligence technique that tries to create a model that maps inputs into outputs. There are two parts to machine learning: training, where one applies the principles of a particular machine learning model on data to create the mathematical mapping function, and the testing component, where one tests the predictive ability of the model on data with known outcomes. A 5-fold cross-validation was done with testing and training. The dataset was randomly divided into a training set that consisted of 18,400 PR and RT sequences and a testing dataset that consisted of 4,600 PR and RT sequences. This was done five times, and an average of the accuracies was calculated.
Feature selection was chosen as the machine learning technique because it helps determine the importance of each of the inputs into the model, and in that way, the importance of each mutation in determining HIV drug resistance may be calculated.
Feature selection (ReliefF), MODTree filtering, FCBF filtering, and CFS filtering were used to determine the importance of each mutation in predicting HIV drug resistance. ReliefF is a component for automatic variable selection in a supervised learning task, which can handle both continuous and discrete descriptors. FCBF filtering is a fast correlation based filtering approach. CFS filtering is a correlation based filtering approached whereas MODTree is a multivalued oblivious decision tree approach.
The open-source software used to perform the machine learning is called Tanagra, version 1.4 [
15]. PR and RT sequences were used separately when determining the importance of particular mutations using these feature selection techniques. The results of the filters were combined additively, and the top 10 mutation positions were calculated for each ARV drug that mathematically had the biggest contribution to predicting HIV drug resistance. These created the association matrix for each drug.
3. Effectiveness of the ANRS with the Incorporation of the Association Matrix
The association matrix for each drug was added to the rules of the ANRS algorithm. This new model was then applied to a separate (unused in the training process) testing dataset, and the changes in the ability to predict HIV drug resistance was analyzed. Changes in the accuracy, sensitivity, and specificity were calculated.
III. Results
The feature selection algorithms were successfully run, and mutations that do not exist in the ANRS rules were found to contribute to resistance for all 10 ARVs tested: IDV (indinavir), LPV (lopinavir), NFV (nelfinavir), SQV (saquinavir), TPV (tipranavir), ABC (abacavir), DDI (didanosine), EFV (efavirenz), NVP (nevirapine), and TDF (tenofovir). These mutations are listed in
Table 1. To determine the difference in predicating HIV drug resistance, the correctly and incorrectly classified sequences were calculated shown in
Tables 2 and
3.
Tables 4 and
5 present the results on the sensitivity and specificity that were calculated to further investigate the effectiveness of adding the association matrix to the ANRS original rules.
This study showed considerable improvement in predicting HIV drug resistance using machine learning against the gold standard ANRS.
IV. Discussion
The results show that the ANRS gold standard can be improved with respect to predicting HIV drug resistance for all ten ARV drugs tested.
Table 1 lists the mutations not present in the ANRS algorithm for each of the 10 ARV drugs. Some of the major contributors to predicting HIV drug resistance for protease ARV drugs, using the feature selection algorithms, were P63, P57, P82, and P69. However, the ANRS algorithm only has P82 in its major mutation list. Similarly, P30, P35, P142, and P83 were identified as important mutations for RT ARV drugs, which were not a part of the ANRS algorithm. Up to the ten most important mutations for each ARV, which were not present in the ANRS algorithm, were combined with the original ANRS algorithm to determine if there were any changes to the ability of the new algorithm to predict the susceptibility and resistance to ARV drugs.
Eighty-five PI sequences (59%) were supposed to be interpreted as resistant but were classified as susceptible according to the original ANRS. Adding the rules derived from the machine learning algorithm results in an 88% ± 7.1% improvement in the overall accuracy. A t-test was performed to determine if the improvement was due to random chance, and a p < 0.001 was obtained. This indicates that there is a statistically significant improvement in the prediction of the susceptibility measure for the five PR drugs.
Thirty-one PI sequences (63%) were wrongly classified as susceptible instead of resistant according to the original ANRS algorithm. Adding the rules derived from the machine learning algorithm, results in an 83% ± 12.1% improvement in the overall accuracy. A t-test was performed to determine if the improvement was due to random chance, and a p < 0.004 was obtained. This indicates that there is a statistically significant improvement in the prediction of the resistant measure for the five PR drugs.
Nearly 130 RT sequences (58%) were wrongly classified as susceptible instead of resistant. Using the machine learning algorithm rules results in a 69% ± 10.9% improvement in the overall accuracy. A t-test was performed to determine if the improvement was due to random chance, and a p-value of 0.004 was obtained. This indicates that there is a statistically significant improvement in the prediction of the susceptibility measures for the five RT drugs.
One hundred forty-seven RT sequences (60%) were wrongly classified as susceptible instead of resistant. Adding the rules derived from the machine learning algorithm results in an 80% ± 5.9% improvement in the overall accuracy. A t-test was performed to determine if the improvement was due to random chance, and a p < 0.004 was obtained. This indicates that there is a statistically significant improvement in the prediction of resistant measures for the five RT drugs.
Table 4 shows the positive predictive value (PPV) and negative predictive value (NPV) of all the PR ARVs used in the study. A Z-score >1.98 was obtained when the new ANRS rules where used indicating there is a statically significant difference when adding the association matrix.
Table 5 shows the PPV and NPV of predicting HIV resistance for PR ARV drugs for both the ANRS algorithm alone and when the machine learning mutations are incorporated into them. The PPV improved by 27% while the NPV improved by 16%. These results show that the incorporation of the machine learning mutation does positively influence the ability of ANRS to predict RT ARV drug resistance.
In conclusion, the above study shows that there is a significant improvement in the prediction ability of the ANRS gold standard. On average, the ANRS algorithm was improved by 79% ± 6.6%. The positive predictive value improved by 28%, and the negative predicative value improved by 10%. Some of the major contributors to predicting HIV drug resistance for protease ARV drugs, using the feature selection algorithms, were P63, P57, P82, and P69. Similarly, P30, P35, P142, and P83 were identified as important mutations for RT ARV drugs. These indicate that the ANRS gold standard has its limitations, which can be improved. Future studies may include using other machine learning algorithms like support vector machines and Bayesian networks. A larger dataset will be of benefit.
Figures and Tables
Table 1
Important mutations that contribute to the resistance of each ARV


Table 2
The correctly classified sequences out of the total sequences and the accuracy in percentage for all the PR ARVs
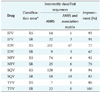

Table 3
The correctly classified sequences out of the total sequences and the accuracy in percentage for all the RT ARVs
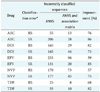

Table 4
PPV and NPV for all the PR ARVs used in the study
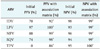

Table 5
PPV and NPV of predicting HIV resistance for PR ARV drugs for both the ANRS algorithm alone and when the machine learning mutations are incorporated into them







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download