Abstract
Objectives
The monitoring of medication compliance in clinical trials is important but labor intensive. To check medication compliance in clinical trials, a system was developed, and its technical feasibility evaluated.
Methods
The system consisted of three parts: a management part (clinical trial center database and a developed program), clinical trial investigator part (monitoring), and clinical trial participant part (personal digital assistant [PDA] with a barcode scanner). The system was tested with 20 participants for 2 weeks, and compliance was evaluated.
Results
This study developed a medication compliance monitoring system that used a PDA with a barcode scanner, which sent reminder/warning messages, logged medication barcode data, and provided compliance information to investigators. Registered participants received short message service (SMS) reminder/warning messages on their PDA and sent barcode data at the dosing time. The age range of the participants was 29 to 73 years. Five participants were <50 years old and 8 were ≥65 years old. The total mean compliance rate was 82.3%. The mean compliance rate was 83.1% in participants <65 years old and 81.1% in those ≥65 years old.
Medication compliance is important in clinical trials for evaluating drug efficacy and safety as well as researching outcomes. Therefore, mediation compliance is monitored by clinical trial investigators by, for example, telephone, text message, and patient diaries. Medication compliance at home may be poorer than that in hospitals or clinical trial centers due to a lack of reminders. Short message service (SMS) reminders increase medication compliance, including in elderly patients [123].
Electronic data capture (EDC) is defined as the collection of clinical trial data on paper (e.g., case report forms, patient diaries, and patient reports) into electronic form. EDC using mobile digital technologies enables clinical trials to be performed more rapidly and at a lower cost [45]. Real time direct data capture in clinical trials enhances EDC by increasing the data accuracy and reducing costs [67]. The use of novel digital technologies in clinical research trials reportedly improves research outputs [5]. Various digital technologies can be used from participant recruitment to data analysis. Personal digital assistants (PDAs) are mobile digital devices that can be used for direct data capture in clinical trials and have a telephone function.
Medication compliance monitoring is important but time-consuming and laborious. In this study, a medication compliance monitoring system using a PDA with a barcode scanner was developed to improve medication compliance and to produce high-quality data in a timely manner. The system enabled real-time monitoring of medication compliance and communication between investigators and participants in clinical trials.
The medication compliance monitoring system (Figure 1) consisted of a management part (clinical trial center database [CTC DB] and software), clinical trial investigator part (monitoring), and clinical trial participant part (PDA).
The CTC DB included a clinical trial list, investigator information (e.g., principle investigator, sub-investigators, and clinical research coordinators), and participant information. The database was linked to our hospital prescription system to obtain information regarding prescription medication, which included the dosage interval (e.g., three times per day, every 8 hours) and duration (medication start to stop dates). Barcodes were attached to medication-containing bags to collect and respond to data in real-time (automatic decision support system), send reminder or warning messages to participants and investigators, and to display information on medication compliance.
The monitoring part registered a trial, assessed the medication compliance of the participants, and enabled investigators to intervene, send messages, and alter medication plans.
Three PDAs with barcode scanners, the MB7000 (Daishin Information Communications, Seoul, Korea), MC-63005 (M3 Mobile, Seoul, Korea), and SCH-M480 (Samsung, Suwon, Korea), were used. The barcode scanner can read oneand two-dimensional barcodes. Barcode information was sent to the CTC DB and stored in the PDA automatically.
The usability of the system was tested with 20 individuals. This study was approved by the Institutional Review Board of Inje University Busan Paik Hospital, Busan, Korea (IRB No. 08-116). A barcode was attached to a bag containing medication. Each barcode contained a participant identification number and information on the medication dosage. Each bag contained two sugar-filled capsules. Participants received an automatic reminder message when they should take the medication and scan the barcode using the PDA, to transfer the barcode information to the CTC DB. The study duration was 2 weeks, during which participants took the medication three times per day at intervals of ≥ 4 hours or at 8 AM, 12 PM, and 6 PM. The data were both transferred to the CTC DB and stored in the PDA in case of a problem with the wireless network. Compliance rates were evaluated based on the CTC DB data. The participants were trained to use the PDA prior to the study. Participants provided feedback regarding the usability of the system at the end of the study.
A clinical trial medication compliance monitoring system was developed at a university hospital clinical trial center. Participants registered for an IRB-approved clinical trial and were prescribed a trial drug according to the approved protocol. The participants were trained to use the PDA and to send the barcode data to the hospital database when required (Figure 1). The system enables clinical trial investigators to check compliance and automatically generates a list of participants with poor compliance. Moreover, the system can send SMS reminder/warning messages to participants and investigators. Participant medication records were stored in the CTC DB and displayed as compliance rates (Figure 2).
All but one of the participants resided in Busan. The age range of the participants was 29–73 years, and 8 of the 20 participants were males (Tables 1, 2). Five participants were <50 years old and 8 were ≥65 years old. The total mean compliance rate was 82.3%. The mean compliance rates between males (mean age, 57.1 years) and females (mean age, 56.3 years) were 86.1% and 79.8%, respectively, with the men having a superior compliance rate than that of the females.
The mean rates of compliance with the predetermined intervals and predetermined times for the day regimens were 83.1% and 81.5%, respectively (Table 2). The mean compliance rates were 83.1% and 81.1% in participants <65 and ≥65 years old, respectively. One participant was unfamiliar with cellular telephones and computers; her compliance rate was 54%. The other participants had used a cellular telephone for ≥4 years (mean, 7.4 years). The results are summarized in Table 1, and the characteristics of the participants and the compliance rates are listed in Table 2. The medication compliance monitoring system generally performed well. The participants only had a few problems with the system, most of which were related to connecting to the Internet and scanning the barcodes. Most of the participants and investigators were satisfied with the system. Errors in using the PDA were associated with the level of experience using a computer, cellular phone, and email but not with age. Most of the participants quickly became accustomed to using the PDA. Two participants required >10 training sessions and had low compliance rates.
This study developed a PDA-based clinical trial medication compliance monitoring system with remote data capture. The overall compliance rate of the system was 82%.
PDAs are not owned by everyone, particularly in the elderly population; however, the monitoring system could, if necessary, be developed as a smartphone application. The PDA based system can be used by non-smartphone owners or individuals reluctant to use their own smartphones. The system reduced the frequency of errors and thus saved time. Moreover, the PDA enabled participants to contact the clinical trial investigator directly. However, this system could not identify if the participants took their medication before scanning the barcode. This study involved 20 participants from Busan and a neighboring city but was limited to a single clinical trial center. This limits the generalizability of the results as other centers may have patients with different characteristics and/or different technical infrastructures. The results for a regimen of three times per day at ≥4 hour intervals were slightly better than those for the other regimen (at 8 AM, 12 PM, and 6 PM), likely because reminder messages were sent at 30-minute intervals until the medication was taken.
A cross-over questionnaire survey of patients with irritable bowel syndrome showed that paper- and PDA-based data capture were equally efficacious [8]; however, the PDA reduced the time and cost of data processing. A study on a wireless hand-held electronic diary and paper diary in Parkinson's disease patients for at least 5 years reported compliance rates of 88% and 98%, respectively; however, compliance with the time schedule was 78% for the paper diary [9]. The hand-held electronic diary increased the compliance rate among patients with a median age of 65 years. Therefore, the use of a hand-held computer facilitates the collection of data at the correct time, irrespective of the participant age.
In a questionnaire-based study, participants aged 63–93 years used a PDA with a barcode scanner to respond to questions. The results support the feasibility of PDA-based systems for monitoring the medication compliance of elderly patients [10].
A pilot study on a real-time electronic adherence monitoring device (EAMD) with SMS reminders involving patients who failed second-line antiretroviral therapy showed a modest improvement in compliance [11]. EAMDs provide precise data on medication compliance but are expensive and inconvenient [12]. Participants in the above mentioned study on the antiretrovirals stated that the device was too large to carry, and it would be better to combine the reminders with the monitoring device [11]. Kalantarian et al. [1314] developed an algorithm that involved use of a necklace (swallows) and smartphone (hand motions) to assess medication compliance. Video (or virtually) observed therapy using videophones connected to telephone landlines and video-enabled mobile cellular devices improved medication compliance in tuberculosis patients [15].
Regulatory authorities may be reluctant to allow the use of new technologies in clinical trials due to concerns regarding data security [5]. However, these problems will be overcome, and new technologies will be implemented in future clinical trials.
These study results indicate the feasibility, usability, and effectiveness of the PDA-based clinical trial medication compliance monitoring system. Use of this system will improve the quality of clinical trials.
Figures and Tables
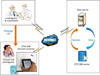 | Figure 1The PDA-based clinical trial drug compliance monitoring system. The system consists of a management part (clinical trial center database [CTC DB] and software), clinical trial investigator part (monitoring), and clinical trial participant part (PDA). |
 | Figure 2The drug compliance management and monitoring system. Participant data are stored (upper right panel) and displayed as a summary and bar chart (left upper and lower panels). A reminder or warning message could be sent to participants (right lower panel). |
Acknowledgments
This research was supported by a grant from the Korea National Enterprise for Clinical Trials (KoNECT) through the Korea Health Technology R&D Project, Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. A070001). I would like to thank Gwangseok Baek for technical support and Su-Jin Jung (Inje University Busan Paik Hospital Clinical Trial Center) for assistance with the trial.
References
1. Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (Weltel Kenya1): a randomised trial. Lancet. 2010; 376(9755):1838–1845.

2. Pernell BM, DeBaun MR, Becker K, Rodeghier M, Bryant V, Cronin RM. Improving medication adherence with two-way short message service reminders in sickle cell disease and asthma: a feasibility randomized controlled trial. Appl Clin Inform. 2017; 8(2):541–559.
3. Huang CY, Nguyen PA, Clinciu DL, Hsu CK, Lu JR, Yang HC, et al. A personalized medication management platform (PMMP) to improve medication adherence: a randomized control trial. Comput Methods Programs Biomed. 2017; 140:275–281.

4. van Dam J, Omondi Onyango K, Midamba B, Groosman N, Hooper N, Spector J, et al. Open-source mobile digital platform for clinical trial data collection in lowresource settings. BMJ Innov. 2017; 3(1):26–31.

5. Andriesen J, Bull S, Dietrich J, Haberer JE, Van Der Pol B, Voronin Y, et al. Using digital technologies in clinical hiv research: Real-world applications and considerations for future work. J Med Internet Res. 2017; 19(7):e274.

6. Welker JA. Implementation of electronic data capture systems: barriers and solutions. Contemp Clin Trials. 2007; 28(3):329–336.

7. Turner D. Digital data capture for electronic patient record systems. J AHIMA. 1995; 66(3):63–65.
8. Bushnell DM, Reilly MC, Galani C, Martin ML, Ricci JF, Patrick DL, et al. Validation of electronic data capture of the irritable bowel syndrome: quality of life measure, the work productivity and activity impairment questionnaire for irritable bowel syndrome and the EuroQol. Value Health. 2006; 9(2):98–105.

9. Nyholm D, Kowalski J, Aquilonius SM. Wireless realtime electronic data capture for self-assessment of motor function and quality of life in Parkinson's disease. Mov Disord. 2004; 19(4):446–451.

10. Boissy P, Jacobs K, Roy SH. Usability of a barcode scanning system as a means of data entry on a PDA for selfreport health outcome questionnaires: a pilot study in individuals over 60 years of age. BMC Med Inform Decis Mak. 2006; 6:42.

11. Evans D, Berhanu R, Moyo F, Nguweneza A, Long L, Fox MP. Can short-term use of electronic patient adherence monitoring devices improve adherence in patients failing second-line antiretroviral therapy? Evidence from a pilot study in Johannesburg, South Africa. AIDS Behav. 2016; 20(11):2717–2728.

12. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999; 21(6):1074–1090.

13. Kalantarian H, Motamed B, Alshurafa N, Sarrafzadeh M. A wearable sensor system for medication adherence prediction. Artif Intell Med. 2016; 69:43–52.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download