Abstract
Objectives
New Zealand is currently implementing a standard for the electronic referral of patients from primary care to District Health Board (DHB) provided specialist services (eReferral). Medical Officers of Health working within DHB public health services receive referrals through a legally mandated disease notification system. Although laboratories have reported notifiable diseases electronically since 2007 clinical and risk factor information are still reported by fax or telephone. This paper describes a project that aims to adapt eReferral for public health purposes.
Methods
A work group of Medical Officers of Health was convened to develop criteria for priority disease selection and to develop data and functional requirements.
Results
Eleven out of 52 notifiable diseases were selected based on potential to improve public health response and or make referral easier for medical practitioners. In addition to identifiers and demographics data requirements included: symptom onset date, occupation and place of work (or other day time location) and workplace name. The work group specified that most enteric disease eReferrals should be triggered by a positive laboratory test. Vaccine preventable disease eReferrals should occur at the time of relevant laboratory test order.
Conclusions
The project is at an early stage and consultation with referrers has been limited. The next stage will require working closely with referring doctors to resolve practical issues with occupation coding, to minimize practice workflow change, and to maintain consistency with other eReferral processes.
Mandatory physician reporting of specified "notifiable" diseases has been required by law in New Zealand for over 100 years. The Public Health Act of 1900 (section 26) required that medical practitioners (and pharmacists) notify cases of specified infectious diseases such as small-pox or enteric fever. The notifier was required to complete the specified form and send it by post to the local District Health Officer.
Subsequent public health legislation has retained legal requirements for notifiable disease reporting and 52 conditions are currently "notifiable" to a Medical Officer of Health in New Zealand [1]. Medical Officers of Health work within the Public Health Services provided by District Health Boards but in some instances a Medical Officer of Health may be responsible for more than one district. Telephone and fax reporting have superseded notification by post in practice although this is not required by law.
The primary purpose of disease notification has been to ensure that other persons at risk of developing the disease are protected by local public health control measures. Over time the aggregation and epidemiological analysis of notifiable disease case reports has come to be seen as equally important at both local and national level. Data are analysed to identify common source outbreaks or important risk factors, to develop and target population level interventions, and to evaluate the effectiveness of those interventions.
For some notifications, such as sporadic cases of enteric disease in settings where the risk of further transmission is low, control measures are limited to providing health advice. In these cases the primary function of reporting is to facilitate outbreak detection and other data analyses. AIDS notifications do not include personal identifiers or contact information and incident cases are reported for aggregate data analysis only.
After the introduction of the 1956 Health Act, data were collected at the national level through a mailed card system until the late 1980s. Then local public health offices began to send diskettes to the national surveillance provider (the New Zealand Communicable Disease Centre) for aggregation into a national database. Subsequently other aggregation methods, such as email of encrypted local database updates, were used until a secure web-based real time national database (EpiSurv 7) was deployed in 2007. This system enables Medical Officers of Health or other public health staff to record details from notifying doctors in the national database thereby providing real time access to data for analysis at the national level.
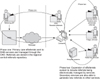
In 2007 a legal requirement for reporting of notifiable diseases by laboratories was introduced [2]. Although the law permits laboratories to report cases manually (e.g., by phone to the Medical Officer of Health) in most cases the requirement is met through Health Level 7 (HL7) messaging from laboratory information systems to EpiSurv (currently 7.2.7). New laboratory-reported cases are visible to local public health staff members who are then able to reconcile lab information with existing physician notification reports using a national identifier (National Health Index [NHI] number). If a corresponding physician report record does not already exist within the database a new case record is generated. These developments have effectively resulted in the electronic reporting of disease cases confirmed by a laboratory test (Figure 1) and have significantly reduced under-reporting in diseases that are diagnosed primarily by a laboratory test.
However significant shortcomings remain within the notifiable disease reporting system. For example, health service requirements for notifiable diseases are defined in a national manual [3], but in practice resource limitations require local services to prioritise those cases deemed to pose the greatest risk to the public. It is likely that reporting doctors are unclear of criteria used for these assessments and may not collect required information at the time of the patient visit.
This issue is compounded by the fact that electronic laboratory reports do not include the clinical or risk factor information that a Medical Officer of Health requires to assess case priority. This information must therefore be supplied separately by phone or fax even for laboratory-reported cases. The laboratory reporting guideline proposed that these data elements could be supplied electronically with laboratory test orders [2]. Such an approach remains feasible but would require that additional data fields be made mandatory in laboratory test requests.
Other issues concern the reporting process itself. For example public health services generally do not formally acknowledge disease notifications. This leaves notifiers uncertain as to whether notification has been received and unclear about what actions public health will or will not undertake. Phone reporting is often not available at night time which is inconvenient for doctors wanting to report cases out of hours and may create unnecessary delays in public health follow-up. It is also possible that, as more doctors become aware that laboratories are reporting electronically, they will become increasingly reluctant to report cases manually.
This paper describes a project being undertaken to redefine the disease notification process as a referral to a specialist service. The proposed new process aims to mitigate some of the shortcomings listed above. The application adapts a newly developed eReferral system that electronically transmits the information required for referral of patients by primary care physicians to District Health Board specialist services.
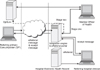
The New Zealand National Health IT plan [4] includes a continuum of care work stream that focuses on the transfer of health information between sector systems using standardised content, process and transfer protocols. The work stream includes the an eReferrals project [5] along with the development of an online forms standard [6] which has been used for the "Care Connect" eReferrals project (Figure 2). This implementation is currently operating within the Auckland region and scheduled for implementation in Hawke's Bay this year.
In the first phase a central referrals office (CRO) has been required to process eReferrals manually. This phase did not meet requirements for real time eReferral of notifiable disease.
A second phase currently under development enables specialist assessment and prioritisation of eReferrals along with response to referring clinicians in real time. Electronic Health Records are almost universally deployed in New Zealand primary care physician offices and at the time of writing most primary care physicians in Auckland now have access to an eReferral module within their Electronic Health Records (or practice management system). The eNotification application adapts the Phase Two eReferral process (Figure 3) as this phase enables real time review of eReferrals by a triage clinician.
Two stages are proposed for the implementation of eNotification. In stage one the Medical Officer of Health, or another staff member from the local public health office, would review eReferrals within the District Health Board eReferral system. In the second stage the eReferrals integration engine would redirect the eReferral message to the national EpiSurv database where it could be accessed by the local public health office.
For those patients for whom no local control actions are required referrers will be advised of this and patients will not receive follow-up services unless subsequent data analysis identifies them as being part of an outbreak.
A work group of Medical Officers of Health from each region was established to develop more specific requirements for eReferral of notifiable diseases. The objectives of the Medical Officer of Health work group were to identify: 1) a set of criteria for selecting notifiable diseases for eReferral, 2) a list of priority diseases, and 3) data items required and data standards.
The criteria for selecting notifiable diseases for eReferral are shown in Table 1. These criteria were based on potential to enhance public health response and to assist doctors' workload.
Using the criteria in Table 1, the work group selected eleven out of the 52 diseases for inclusion in the eNotification system. Table 2 shows the diseases selected along with the trigger event and symptom for which the onset date should be recorded.
For the first six enteric diseases listed the referral trigger event is the receipt of a positive test result. A clinical diagnosis cannot be made with certainty and there is no pressing need for medical treatment of exposed contacts.
By contrast, for hepatitis A and the vaccine-preventable diseases it was decided that referral should occur at the time the diagnosis is first considered by the doctor. This is for two reasons. These diseases can often be diagnosed clinically (although laboratory confirmation is still recommended). More importantly in these diseases antibiotic treatment or vaccination of exposed contacts must be done as soon as possible to be effective.
The onset date of symptoms is a crucial piece of information in public health management of diseases. The key symptom required for recording onset date was specified for each disease.
In addition to symptom onset date, other information is important to public health follow-up. These data items were identified for all eReferrals to public health (Table 3). It was expected that demographic data would be derived from the patient NHI although if unavailable these data (name, age, sex, ethnicity) would need to be generated from the referring doctor's practice management system. Occupation may not be routinely recorded within general practitioner (GP)-based Electronic Health Records and is not part of the NHI. This data field however was however considered to be critical for useful eNotification. For the six conditions triggered by a positive laboratory result, the occupation and related data fields will therefore need to be completed at the time of laboratory test request and stored for transmission in the event of a positive test result.
The advent of electronic patient referral has created the opportunity
to improve the current New Zealand notifiable disease referral system. Detailed requirements have been identified for eReferral to public health (eNotification) and can now be implemented in the next phase of eReferral implementation.
The project is at an early stage however and the next phase will focus on resolving practical issues associated with occupational coding in the medical practice. A recent evaluation of eReferral in New Zealand highlighted the importance of working with referrers [8]. It will be important to work with to identify ways in which eNotification can be devised so that there is minimal need for change to practice workflow and at the same time consistency with other eReferral processes.
The implementation of eReferral will enhance awareness among referring doctors of the follow-up services provided by public health. As the project progresses in consultation with general practitioners and other medical referrers will develop a better understanding of the criteria used by public health for follow-up actions and the importance of providing information such as occupation and onset date. Providing practical issues can be resolved eNotification will replace phone or fax notification and reduce referrer workload.
Figures and Tables
Figure 1
Current New Zealand notifiable disease reporting processes. EpiSurv: a secure web-based real time national database, HL7: Health Level Seven.

Figure 2
The care connect Auckland eReferral system [7]. GP: general practitioner, PMS: practice management system, CRO: central referrals office, Concerto: eReferral portal, DHB: District Health Board.
Acknowledgments
The authors would like to thank other members of the Medical Officers of Health workgroup: Dr. Daniel Williams, Dr. Clair Mills, Dr. Jill McKenzie, Dr. Phil Shoemack, Dr. Jonathan Jarman, and Dr. Annette Nesdale. Thanks also to Corrine Gower from Waikato DHB, Grant Ramsay from healthAlliance, and Ian Hight from HealthLink.
References
1. New Zealand Ministry of Health. Notifiable diseases: diseases that are notifiable to the medical officer of health [Internet]. c2012. cited at 2012 Aug 20. Wellington: Ministry of Health;Available from: http://www.health.govt.nz/our-work/diseases-and-conditions/notifiable-diseases.
2. New Zealand Ministry of Health. Directory laboratory notification of communicable diseases: national guidelines. 2007. Wellington: Ministry of Health.
3. New Zealand Ministry of Health. Communicable disease control manual 2012. 2012. Wellington: Ministry of Health.
4. New Zealand National IT Health Board. Enable an integrated healthcare model. 2010. Wellington: Ministry of Health.
5. New Zealand National IT Health Board. Continuum of care: eReferrals [Internet]. c2010. cited at 2012 Sep 15. Wellington: Ministry of Health;Available from: http://www.ithealthboard.health.nz/content/continuum-care-ereferrals.
6. New Zealand National IT Health Board. Online forms architecture technical specification. 2010. Wellington: Ministry of Health.
7. Care Connect eReferrals. eReferrals rollout [Internet]. 2012. cited at 2012 Sep 15. Auckland: healthAlliance;Available from: http://www.ereferrals.co.nz/NEWS/NewsletterApril2012/tabid/221/Default.aspx.
8. Warren J, Gu Y, Day K, White S, Pollock M. Electronic referrals: what matters to the users. Stud Health Technol Inform. 2012. 178:235–241.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download