Sleep is an essential life process in u-health environment [
1]. It is as important to our well-being as the food we eat, the water we drink, and the air we breathe. Lack of sleep is a common feature of our society, affecting students and adults alike [
2]. Problem sleepiness can be deadly. Approximately 140,000 automobile crashes each year in Korea result from drivers who were "asleep at the wheel [
3]." The analysis of sleep stage based on biosignal is the way to evaluate the quality of sleep [
4]. Identification of an individual's sleep stage is the first step in sleep studies for clinical diagnosis and treatment of sleep disturbances. It is common knowledge that sleep is an essential state of rest and refreshment. Sleepiness, trouble concentrating, and increased risk of accidents are caused by insufficient sleep, which can be better understood by interpreting sleep stages and the sleep cycle The theory of non-linear dynamic systems, also called chaos theory, has now progressed to a stage, where it becomes possible to study self-organization and pattern formation in the complex neuronal networks of the brain [
5]. The chaotic process, correlation dimension (
D2) and largest Lyapunov exponent (
L1), were performed to quantify the complexity of physiological phenomena of sleep electroencephalography (EEG) at different sleep stages.
D2 proved to be very useful for characterizing the brain dynamics in different sleep stages. It is found that
D2 decrease in deep sleep stages, thus reflecting a synchronization of EEG [
6,
7].
D2 was also used for characterizing the brain dynamics doing mental tasks [
8-
10]. The
D2 was also used for characterizing the nature of EEG signal. In principle, converging
D2 value point towards a non-linear deterministic nature and diverging
D2 values would stress the interpretation of EEG signals as noise. The next section presents the experimental results of the performance of nonlinear analysis and discussion.
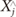 , is found by searching for the point that minimizes the distance to the particular reference point, Xj, This is expressed as
, is found by searching for the point that minimizes the distance to the particular reference point, Xj, This is expressed as




 PDF
PDF ePub
ePub Citation
Citation Print
Print


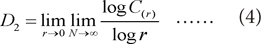
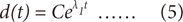




 XML Download
XML Download