Abstract
Figures and Tables
 | Figure 1Control of close contacts of a laboratory-confirmed MERS-CoV infection case.MERS-CoV, middle east respiratory syndrome coronavirus; PCR, polymerase chain reaction; MERS, middle east respiratory syndrome.
|
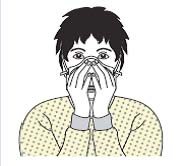
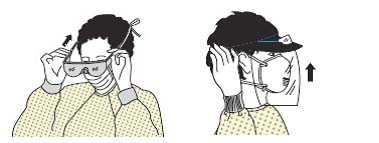
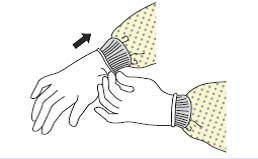
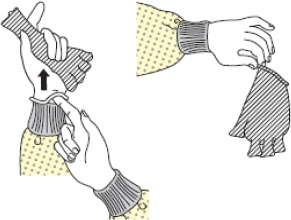
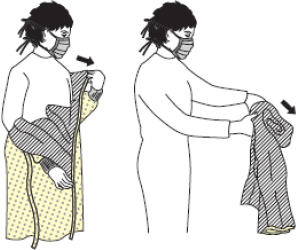
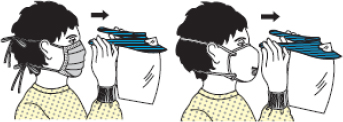
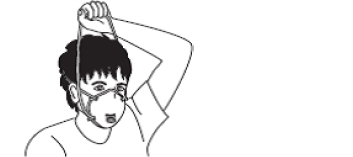
 | Figure 2A schematic of the procedures for donning/doffing of PPE depending on the physical space arrangement.PPE, personal protective equipment
|
 | Figure 3Triple-packaging of diagnostic specimens (Category/Package: primary container, secondary container, outer package). |
 | Figure 4Movement of the autopsy team: taking off shoes and clothes and wearing a surgical gown and other PPE in the dress-in room (1), entering the autopsy room (2), taking off PPE and performing hand hygiene in the dress-out room (3), re-entering the dress-in room to put on the shoes and clothes (4) (adapted from WHO guideline [11]).PPE, personal protective equipment.
|
Table 1
Strength of recommendation and quality of evidence for recommendation

Table 2
Composition of a MERS infection emergency committee

Table 3
Time required for infectious agent removal based on the number of air changes per hour (adapted from CDC guideline [28])
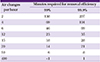
| Air changes per hour | Minutes required for removal efficiency | |
|---|---|---|
| 99% | 99.9% | |
| 2 | 138 | 207 |
| 4 | 69 | 104 |
| 6 | 46 | 69 |
| 12 | 23 | 35 |
| 15 | 18 | 28 |
| 20 | 14 | 21 |
| 50 | 6 | 8 |
| 400 | <1 | 1 |
Table 4
Definitions of MERS-CoV infection cases

aThe Middle East region includes the Arabian Peninsula and its neighboring countries (Bahrain, Iraq, Iran, Israel and the West Bank, Gaza, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Syria, UAE, and Yemen).
bClose contact refers to a case of direct contact with droplets a MERS patient without wearing appropriate personal protective equipment (gown, gloves, highly efficient mask, goggles or face shield) and/or staying within a 2 m distance or in the same interior space with a patient.
cMERS outbreak refers to two or more cases of laboratory-confirmed MERS-CoV infection in the same healthcare facility.
Table 5
Risk assessment and recommendations for asymptomatic MERS contacts

High-risk close contact: contact during an aerosol-generating procedure (e.g. nebulizer, intubation, endotracheal suction, bronchoscopy, etc.). Intermediate-risk close contact: contact within 2 m distance of a laboratory-confirmed MERS patient or a stay at the same ward/floor of a hospital exposed to laboratory-confirmed MERS patients. Casual contact: brief contact with >2 m distance from a laboratory-confirmed MERS patients.
MERS, Middle East Respiratory Syndrome.
Table 6
Control of visitors to Middle East countries or healthcare facilities affected by MERS outbreaksa depending on symptom manifestations

MERS, Middle East Respiratory Syndrome; PCR, polymerase chain reaction.
aA healthcare facility with two or more cases of laboratory-confirmed MERS-CoV infection is regarded as being affected by MERS outbreak.
bIn the presence of pneumonia, the patient is classified as a patient with suspected MERS-CoV infection and placed under inpatient quarantine care.
Table 7
PPE donning procedure (adapted from Healthcare Infection Control Practices Advisory Committee guideline [35])

Table 8
PPE doffing procedure (adapted from Healthcare Infection Control Practices Advisory Committee guideline [35])

Table 9
PPE application for different situations

Table 10
Risk assessment of a suspected/confirmed MERS-CoV infection case detected in the dialysis room

Table 11
Methods to reduce aerosol generation during an autopsy

Table 12
Application scope and disinfection method depending on sterilization and disinfection levels

Table 13
Disinfection and sterilization of respiratory therapy equipment
Acknowledgment
Notes
Conflict of Interest This guideline was developed with the support of the Korean Society of Infectious Diseases, Korean Society for Healthcare-associated Infection Control and Prevention, and Korean Association of Infection Control Nurses. We hereby declare that the researchers participating in the development and review of this guideline did not receive any research grant that could potentially influence the finalized guideline and that they were not influenced by any particular interest groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download