Abstract
We report the case of a patient with fulminant myocarditis caused by influenza A virus, who presented with acute-onset heart failure and cardiogenic shock and was treated successfully with single dose of intravenous peramivir and with pharmacologic hemodynamic support. A 45-year-old Korean woman presented to our emergency department (ED) with shortness of breath and an episode of seizure that developed abruptly 5 hours before she arrived in the ED. She had a history of recurrent epileptic seizure 25 years ago, but denied other specific medical illnesses. In the ED, she was hypoxemic (arterial partial pressure of oxygen, 59.8 mmHg on room air) and chest radiography revealed bilateral alveolar infiltrates. A rapid antigen test for influenza A virus was positive, and she was administered a single dose of peramivir (300 mg) intravenously. Five hours later, the patient's dyspnea had worsened and she was hypotensive (blood pressure, 86/53 mmHg), requiring norepinephrine infusion. Further evaluation disclosed an increased cardiac troponin I level of 1.36 ng/mL and a depressed left ventricular ejection fraction of 30%. Under the diagnosis of influenza A-associated myocarditis and cardiogenic shock, she was managed with continuous critical care in the intensive care unit. On day 3, the patient's dyspnea began to resolve and her ventricular function returned to normal. Real-time polymerase chain reaction assays for influenza viruses in serial nasopharyngeal aspirates were positive for influenza A (hH3N2) with a threshold cycle value of 27.39 on day 2, but these became negative by day 4. The patient recovered and was discharged on day 9 after admission. In conclusion, this case indicates that intravenous peramivir might be an effective antiviral agent for the treatment of severe influenza A virus infection.
Influenza viruses cause the most important human respiratory infections. It is estimated that each year, 5% to 10% of adults and 20% to 30% of children globally are infected by an influenza virus, causing substantial incidence of disease, hospitalization, and mortality [1]. Besides respiratory tract infections, influenza viruses occasionally are associated with nonrespiratory complications such as encephalopathy, myocarditis, and myopathy [2]. There has been increased awareness that serious extrapulmonary complications appear to be more common in patients infected with the highly pathogenic avian H5N1 influenza virus and the 2009 pandemic H1N1 influenza virus (H1N1pdm2009) than seasonal influenza viruses.
A spectrum of influenza-associated myocarditis ranges from asymptomatic to fulminant myocarditis, depending on the timing of presentation, the extent of myocardial involvement, and individual factors [3]. Importantly, fulminant myocarditis can result in cardiogenic shock or death; prompt pharmacologic and mechanical circulatory supportive measures are necessary for favorable outcomes. Antiviral therapy with neuraminidase inhibitors (NAIs) is also recommended to limit the rounds of viral replication and prevent disease progression. However, there are still insufficient data about the effect of NAIs on outcomes in potentially fatal cases.
Peramivir (BioCryst Pharmaceuticals Inc., Durham, NC, USA) is a novel intravenous (IV) NAI administered once daily. It was licensed in Japan and the Republic of Korea in 2010. In the United States, IV peramivir is still an investigational NAI, but it was made temporarily available in 2009 for hospitalized patients infected with H1N1pdm2009 under an Emergency Use Authorization [4]. In December 2014, IV peramivir was approved for treatment of acute uncomplicated influenza by the US Food and Drug Administration. While IV peramivir provides a new choice for immediate delivery of an effective single-dose treatment, there are limited data available on peramivir treatment for severe influenza with complications.
Herein, we report an interesting case of influenza A-associated fulminant myocarditis presenting with heart failure and cardiogenic shock that was successfully treated with a single-dose of IV peramivir and with hemodynamic supportive measures.
In March 2015, a 45-year-old Korean woman presented to our emergency department (ED) with shortness of breath and an episode of seizure. Five hours before arriving in the ED, she developed a tonic-clonic seizure for 30 seconds and a high temperature of 40℃. The patient had preceding upper respiratory infection 4 days before coming to the ED. She also complained of severe coughing productive of blood-tinged sputum, and generalized myalgias. Her past medical history was unremarkable except for recurrent (one or two per year) epileptic seizures with oral phenytoin maintenance for 25 years. She had no known allergies. She did not receive the seasonal influenza vaccine.
On physical examination, the patient's blood pressure was 91/59 mmHg, and she had tachycardia (114/min), tachypnea (32/min), and a temperature of 37.9℃. She looked acutely ill, but was mentally alert. Examination of her lungs revealed coarse crackles throughout bilaterally. Other findings were unremarkable. A chest radiograph showed cardiomegaly and bilateral patchy consolidations. An electrocardiogram revealed low QRS voltage in the precordial leads (the entire QRS-complex amplitude was <8 mm). Arterial blood gas analysis revealed pH of 7.38, carbon dioxide tension of 27.7 mmHg, partial pressure of oxygen of 59.8 mmHg, bicarbonate level of 16.7 mmol/L, base excess of 6.4 mmol/L, and oxygen saturation level of 90.7%. The patient was given supplemental oxygen via nasal cannula (2 L/min).
Initial laboratory findings revealed a hemoglobin level of 10.0 g/dL, white blood cell count of 18.8 × 103/mm3 (neutrophils, 85.6%), platelet count of 504 × 103/mm3, blood urea nitrogen level of 14.9 mg/dL, creatinine level of 0.6 mg/dL, and C-reactive protein level of 4.92 mg/L (normal range, 0.0-5.0 mg/L). An influenza rapid antigen test on the patient's nasopharyngeal swab sample, which was promptly performed because of residual seasonal influenza activity in the community, was positive for influenza A virus. She was immediately given a single dose (300 mg) of IV peramivir, and levofloxacin, 500 mg/day.
The patient's dyspnea worsened, however, and she became hypotensive (blood pressure, 86/53 mmHg). She was managed with increased oxygen supply (4 L/min), furosemide, and norepinephrine (0.125 µg/kg/min). Additional blood tests revealed the following component levels: troponin I, 1.36 ng/mL (normal range, 0.0-0.16 ng/mL); creatine kinase-MB (CKMB), 49.27 ng/mL (normal range, 0.0-3.61 ng/mL); creatine phosphokinase (CPK), 1,585 IU/L (normal range, 38-185 IU/L); lactate dehydrogenase, 937 IU/L (normal range, 238-422 IU/L); N-terminal pro b-type natriuretic peptide, 162.8 pg/mL (normal range, 0-125 pg/mL), and myoglobin, 138.8 ng/mL (normal range, 25-58 ng/mL). Serology for hepatitis B virus, hepatitis C virus and HIV were all negative.
A chest computed tomography scan demonstrated multifocal patch consolidations in both lungs, with ground-glass opacities, suggestive of pulmonary edema or atypical pneumonia. Transthoracic echocardiogram showed a depressed left ventricular (LV) ejection fraction of 30%, LV enlargement, diffuse hypokinesia with sparing LV apex, and decreased right ventricular apical wall motion, which was compatible with acute myocarditis (Fig. 1).
The patient was clinically diagnosed with influenza A-associated fulminant myocarditis, resulting in pulmonary edema and cardiogenic shock, and admitted to the intensive care unit. On day 2 of hospitalization, her hemodynamic state had stabilized and she no longer required norepinephrine to maintain blood pressure within a normal range. A follow-up transthoracic echocardiogram taken on day 3 showed improved ventricular systolic function, with LV ejection fraction at 55% to 60%. Laboratory tests on day 4 revealed a troponin I level of 0.168 ng/mL, CK-MB level of 41.31 ng/mL, and CPK level of 1,459 IU/L. On day 5 of her hospitalization, the patient's dyspnea had improved and supplemental oxygen was stopped. On day 6, she was transferred to a general ward. A follow-up chest computed tomography scan demonstrated improved status of pulmonary edema, with residual, mild bilateral pleural effusion. The patient was discharged with recovery on day 9 after admission. Although her muscle enzyme levels still remained elevated 16 days after discharge (CK-MB, 42.63 ng/mL; CPK, 1,340 IU/L), she had been asymptomatic and carefully observed in the outpatient clinic (Fig. 2).
Monitoring of real-time reverse transcription-polymerase chain reaction assays (Real-Q Flu A, B Detection Kit; BioSewoom Inc., Seoul, Korea) using the patient's nasopharyngeal aspirates showed positivity for both influenza A (threshold cycle value, 27.23) and subtype hH3N2 (threshold cycle value, 27.39) on day 2 (Fig. 3), but became negative by day 4 of the patient's hospitalization.
In this case report, we describe a case of severe influenza A/H3N2 virus infection complicated with fulminant myocarditis. The patient recovered successfully with early administration of IV peramivir and pharmacologic hemodynamic support, without mechanical circulatory intervention.
Influenza infection can cause cardiovascular complications through direct myocardial involvement of the virus as myocarditis, or through exacerbation of existing cardiovascular disease. The frequency of influenza-associated myocarditis has been reported up to 10% [35]. The clinical spectrum of viral myocarditis varies from asymptomatic to fulminant myocarditis resulting in cardiogenic shock and death [36]. Most cases of influenza-associated fulminant myocarditis have been reported in pandemic influenza infection as multiple organ failure or a terminal complication [257]. Among 58 cases of myocarditis with influenza A/H1N1pdm2009 infection reviewed in detail, the proportion of fulminant myocarditis accounted for 62% (n=36), with a mortality rate of 39% (n=14). It has been suggested that development of fulminant myocarditis might depend on the viral pathogenicity or host immunity [7]. On the other hand, fulminant myocarditis occurs occasionally with seasonal influenza infection. There have been only four cases of influenza A/H3N2-associated myocarditis published in the English-language literature since 2000 [891011]. Two of them were fulminant myocarditis; one was rapidly fatal [11] and another required the circulatory assist devices [8]. The majority of previous reports have described that the clinical course of influenza-associated acute fulminant myocarditis needed mechanical ventilation and circulatory assist devices (e.g., intraaortic balloon pumping, extracorporeal membrane oxygenation, percutaneous left ventricular-assist device) and pharmacologic hemodynamic support, despite administration of oral oseltamivir or inhaled zanamivir.
In this case with seasonal influenza A/H3N2-associated fulminant myocarditis, her hemodynamic dysfunction became stabilized within 2 days of illness with supportive catecholamine and diuretics. Moreover, monitoring of real-time polymerase chain reaction assays of influenza viruses on serial nasopharyngeal aspiration samples indicated rapid reduction of viral shedding from upper respiratory tissues. It is assumed that IV peramivir might limit ongoing viral replication effectively and prevent the progression of the disease. Consequently, in this patient with no remarkable underlying diseases except epilepsy, early effective antiviral therapy and the pharmacological hemodynamic support had been shown to be efficacious to control influenza-associated fulminant myocarditis, without requiring circulatory assist devices.
In our case patient who had no previous known heart disease, the echocardiographic findings for her left ventricular dysfunction were mostly helpful for distinguishing between acute myocarditis and stress cardiomyopathy. The typical morphological feature of myocarditis reveals diffuse left ventricular dysfunction, as observed in this case. On the contrary, that of stress cardiomyopathy is systolic dysfunction, selectively involving mid and apical segments of left ventricle, so called apical ballooning.
NAIs such as oseltamivir and zanamivir are established treatments for influenza either orally or by inhalation for patients with influenza. These routes may not provide rapid, reliable drug delivery in seriously ill patients who are unable to tolerate oral or inhaled NAIs, or where absorption of the drug is unreliable [12]. Peramivir is a novel IV NAI that has potent antiviral activity against influenza A and B viruses, and can provide a new choice for immediate delivery of an effective treatment in one dose [412]. Since the IV formulation of peramivir was licensed in the Republic of Korea in 2010, it has been prescribed increasingly as a single IV dose of 300 mg or 600 mg for hospitalized patients with influenza. However, there are insufficient data on the effectiveness and dosing of IV peramivir in treating severe influenza with complications [1213]. In reports on two patients with influenza A-associated fulminant myocarditis who required mechanical circulatory assist, IV peramivir was administered on a different dosing and duration regimen as an adjunct to oral oseltamivir [814].
To our knowledge, this is the first case of influenza-associated fulminant myocarditis that was initially treated with IV peramivir, followed by a return to normal ventricular function within 72 hours. In this case, the patient had shown sustained elevation in serum muscle enzyme levels (i.e., CK-MB level ≥10 times the normal range and CPK more than seven times the normal range) after clinical recovery from influenza and myocarditis. However, she had been asymptomatic without evidence of myositis or seizure activities. One possible explanation is an adverse event of peramivir, as described in a package insert [15].
This case report has some limitations. First, myocarditis was not confirmed by endomyocardial biopsy or detection of influenza viral RNA in heart tissue. We were unable to get pathologic confirmation because of rapid recovery of the patient's clinical course. Second, the presence of either viremia or viral pneumonia remains unclear in considering the pathogenesis of influenza-associated myocarditis. Third, other triggering causes of heart failure cannot be ruled out completely in this case with epileptic seizure. Lastly, other non-infectious and infectious etiologies except influenza viruses were not fully evaluated in this case, because the patient was obviously diagnosed and treated influenza A, followed by improvement.
In conclusion, we report the case of a patient with severe influenza A virus infection complicated with rapidly progressive fulminant myocarditis, who successfully recovered after early use of a single dose of IV peramivir and pharmacologic hemodynamic support. The importance of prompt treatment with the effective NAI is stressed, as well as early diagnosis in patients with severe complicated influenza. Further study is warranted to determine adequate dosing, as well as the effectiveness of IV peramivir for the treatment of severe influenza.
Figures and Tables
Figure 1
Initial radiologic and echocardiographic findings in a patient with influenza A-associated fulminant myocarditis seen in the emergency department. (A) Chest radiography showing bilateral alveolar infiltrates. (B) Chest computed tomography scan showing bilateral nodular ground-glass opacities and consolidation. (C) Two-dimensional echocardiogram demonstrating diffuse hypokinesia with sparing left ventricular apex in the four-chamber view, end-diastolic phase. (D) End-systolic phase.
LA, left atrium; LV, left ventricle.
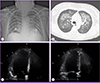
Figure 2
A graph illustrating the clinical course of the influenza-associated fulminant myocarditis case reported in this article, showing changes in clinical findings and supportive measures following the peramivir therapy.
RAT, rapid antigen test; RT-PCR, real-time polymerase chain reaction; CK-MB, creatine kinase-myocardial band.

Figure 3
Results of real-time polymerase chain reaction assays showing amplification curves of the influenza A virus and hH3N2 subtype detection on a nasopharyngeal aspirates collected on day 2 of hospitalization. Two positive control samples for human RNase P were included in the assays.
RFU, relative fluorescence units; C(t), cycle threshold; Flu A, influenza A.

References
1. World Health Organization (WHO). Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012; 87:461–476.
2. Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008; 26:Suppl 4. D59–D66.

3. Estabragh ZR, Mamas MA. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol. 2013; 167:2397–2403.

4. Birnkrant D, Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009; 361:2204–2207.

5. Moore DL, Vaudry W, Scheifele DW, Halperin SA, Déry P, Ford-Jones E, Arishi HM, Law BJ, Lebel M, Le Saux N, Grimsrud K, Tam T. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003-2004. Pediatrics. 2006; 118:e610–e619.

6. Gupta S, Markham DW, Drazner MH, Mammen PP. Fulminant myocarditis. Nat Clin Pract Cardiovasc Med. 2008; 5:693–706.

7. Ukimura A, Satomi H, Ooi Y, Kanzaki Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res Treat. 2012; 2012:351979.

8. Yoshimizu N, Tominaga T, Ito T, Nishida Y, Wada Y, Sohmiya K, Tanaka S, Shibata K, Kanzaki Y, Ukimura A, Morita H, Hoshiga M, Ishizaka N. Repetitive fulminant influenza myocarditis requiring the use of circulatory assist devices. Intern Med. 2014; 53:109–114.

9. Rogers VL, Sheffield JS, Roberts SW, McIntire DD, Luby JP, Trevino S, Wendel GD Jr. Presentation of seasonal influenza A in pregnancy: 2003-2004 influenza season. Obstet Gynecol. 2010; 115:924–929.
10. Ando M, Miyazaki E, Hiroshige S, Ashihara Y, Okubo T, Ueo M, Fukami T, Sugisaki K, Tsuda T, Ohishi K, Yoshitake S, Noguchi T, Kumamoto T. Virus associated hemophagocytic syndrome accompanied by acute respiratory failure caused by influenza A (H3N2). Intern Med. 2006; 45:1183–1186.

11. Nolte KB, Alakija P, Oty G, Shaw MW, Subbarao K, Guarner J, Shieh WJ, Dawson JE, Morken T, Cox NJ, Zaki SR. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000; 21:375–379.

12. Ison MG, Fraiz J, Heller B, Jauregui L, Mills G, O'Riordan W, O'Neil B, Playford EG, Rolf JD, Sada-Diaz E, Elder J, Collis P, Hernandez JE, Sheridan WP. Intravenous peramivir for treatment of influenza in hospitalized patients. Antivir Ther. 2014; 19:349–361.

13. Hernandez JE, Adiga R, Armstrong R, Bazan J, Bonilla H, Bradley J, Dretler R, Ison MG, Mangino JE, Maroushek S, Shetty AK, Wald A, Ziebold C, Elder J, Hollister AS, Sheridan W. eIND Peramivir Investigators. Clinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an Emergency IND program in the United States. Clin Infect Dis. 2011; 52:695–706.

14. Cobas M, Abbo L, Santos M, Baccini-Jauregui C, Pham S. Successful management of fulminant influenza A subtype H1N1 myocarditis. BMJ Case Rep. 2010; 2010:pii: bcr0220102763.

15. Biocryst Pharmaceuticals. Rapivab (peramivir injection) package insert. 2014. Accessed 25 April 2015. Available at: http://www.rapivab.com/prescribing-information/.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download