Abstract
Actinobacillus ureae, formerly known as Pasteurella ureae, is a rare human pathogen. Twenty-eight cases of A. ureae infections in humans have been reported since its first description in 1960. Various predisposing conditions such as skull fracture, alcohol abuse, neurosurgery, schizophrenia, odontal infection, diabetes, HIV infection/AIDS, Waldenström macroglobulinemia, COPD, malnutrition, rheumatoid arthritis, HCV hepatitis, etanercept, or methotrexate have been associated with infections caused by A. ureae. We report the first case, in the medline-based literature, of A. ureae psoas muscle abscess and sepsis in a HBV carrier patient.
Actinobacillus (Pasteurella) ureae is a commensal of the human respiratory tract and various mammals. It is an organism of low pathogenicity and is a rare human pathogen (1). Only 28 cases of A. ureae infections in humans have been reported since its first description in 1960, including meningitis (2), pneumonia (3-6), hepatitis (7), sepsis (7, 8), conjunctivitis (9), otitis media (10), peritonitis (11), endocarditis (12), chronic bronchitis (13), bone marrow infection (14) and septic arthritis (15). Here, we report the first case, in the medline-based literature, of A. ureae psoas muscle abscess and sepsis in a HBV carrier patient.
A 76-year-old male was admitted to the department of internal medicine with fever, lower back pain, and general weakness after suffering from a slip-down injury 25 days before admission while working at his farm. His past medical history was positive for chronic hepatitis B infection, stroke, and hypertension. He had undergone appendectomy and hydrocelectomy. However, he was not a smoker nor drank alcohol.
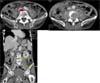
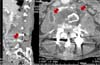
On initial physical examination, he was acutely ill looking and showed confused mentality. His body temperature was 38.1℃, blood pressure was 140/80 mmHg, pulse rate was 96 beats/min, and respiration rate was 20 breaths/min. Positive clinical findings included tenderness and swelling on the lower back. The chest X-ray was unremarkable. The abdominal CT showed L4 vertebral body compression fracture with both psoas muscle abscess (right: 2.7×1.7×1.3 cm, left: 4.3×2.1×2.3 cm) (Fig. 1). Laboratory results were as follows: normochromic normocytic anemia (Hb = 10.2 g/dL, Hct = 30.0%); white blood cell count (WBC), 14.21×109/L (polymorphs, 76.9%; lymphocytes, 9.6%; monocytes, 11.2%; eosinophils; 0.8%); platelet, 427×109/L; erythrocyte sedimentation rate (ESR), 85 mm/h; C-reactive protein (CRP), 12.65 g/L; AST, 279 IU/L; ALT, 142 IU/L; ALP, 198 IU/L; total bilirubin, 0.8 mg/dL; prothrombin time, 20.6 sec (INR=1.88). Other blood biochemistry tests were normal (Table 1). His HBsAg was positive and HBV-DNA was 393,900 copies/mL. The patient was initially treated with intravenous ceftazidime and metronidazole. On the 7th hospital day, the fusion operation of L4-5 vertebral body and incision and drainage was performed. The blood culture was positive for A. urea, which was susceptible to penicillin, amikacin, ampicillin, aztreonam, cefazolin, cefotaxime, ciprofloxacin, gentamicin, and cotrimoxazole. Antibiotics were changed to ceftazidime. Echocardiography showed no vegetation on cardiac valves. A few days after treatment, fever subsided and inflammatory indices such as ESR, CRP, and platelets returned to normal values. Follow up abdominal CT scan after 25 days of treatment showed reduced size of right psoas abscess to 1.0×2.3×1.5 cm and disappeared left psoas abscess (Fig. 2). A repeated blood culture performed on 14 days and 30 days after admission revealed no growth. After 30 days of intravenous antibiotic therapy, he was discharged with oral cefixime for 2 weeks. He was afebrile on control visit in outpatient clinic after 2 weeks.
A ureae is a small, non-motile, vacuolated, bipolarstaining, pleomorphic organism and is gram-negative rod that grows well on media containing blood. The organism is most often a harmless commensal. However, under conditions of immune compromise or disruption of normal physical barriers to infection, it can lead to serious illness. Various predisposing condition such as postsurgical infection, periodontal disease, emphysema, alcohol related cirrhosis, skull fracture, alcohol abuse, neurosurgery, schizophrenia, odontal infection, diabetes, HIV infection/AIDS, Waldenstrom macroglobulinemia, COPD, malnutrition, rheumatoid arthritis, HCV hepatitis, etanercept, or methotrexate have been associated with infections caused by A. ureae (1-15). In this case, chronic hepatitis B was an underlying immune-compromised condition, which is a predisposing factor that is not previously mentioned in literature, and this may impair the defense against the organism. As most of the genus Actinobacillus species are commensal or pathogens of animals, especially cattle, horses, and pigs, history of trauma or recreational activities may provide important clues for establishing early diagnosis (15, 16). This patient had a history of slip-down injury while working at his farm. Therefore, it could be postulated that the origin of A.ureae in this case is from environmental localized mammalian flora.
Since its first description by Minter in 1881 (17), a psoas abscess has been a rare, but life threatening disea.se. The psoas muscle lies in close proximity to organs such as the sigmoid colon, appendix, jejunum, ureters, abdominal aorta, kidneys, pancreas, spine, and iliac lymph nodes. Hence infection in these organs can spread to the iliopsoas muscle. The abundant blood supply of psoas muscle is believed to be a predisposing factor for hematogenous spread from occult sites of infection (18) and this is called primary psoas abscess. About 18-20% of primary psoas abscess is associated with history of trauma (19). Thus this case could also be classified as primary psoas abscess. Staphylococcus aureus is the most common causative organism of primary psoas abscess and Streptococcus species and Escherichia coli are common in secondary psoas abscess (19).
A.ureae strains are susceptible to most antimicrobials including ampicillin, cephalothin, cefoxitin, tetracycline, aminoglycosides, and trimethoprim-sulfamethoxazole. Penicillin is favored as the first line antibiotic for invasive disease, followed by erythromycin and third-generation cephalosporins (15). In this case, the isolate showed susceptibility to all antibiotics and he was successfully treated with ceftazidime.
Other diagnostic modalities are available. Molecular biology-based techniques have proved useful for the detection and identification of organisms that are difficult or impossible to culture in vitro. In addition, analysis of DNA sequences has been used to name organisms that were unidentifiable by phenotypic testing (20).
Of the documented cases in the literature, this is the first case, to our knowledge, of a serious A.ureae infection associated with psoas muscle abscess that has been documented in HBV carrier without progression to liver cirrhosis. When a patient with psoas muscle abscess is found, the possibility that uncommon organism could be the cause of infection should always be seriously considered and effort should be made to identify species and obtain antibiotic susceptibility. This will lead to early initiation of appropriate antibiotic therapy resulting in successful treatment outcome.
Figures and Tables
Fig. 1
L4 vertebral body compression fracture and osteolytic lesions were seen. In both psoas muscles, the elongated hypodense lesions with peripheral enhancement were seen. (right: 2.7×1.7×1.3 cm, left: 4.3×2.1×2.3 cm)
References
1. Kaka S, Lunz R, Klugman KP. Actinobacillus (Pasteurella) ureae meningitis in a HIV-positive patient. Diagn Microbiol Infect Dis. 1994. 20:105–107.

2. de Castro N, Pavie J, Lagrange-Xélot M, Bouvry D, Delisle F, Parrot A, Molina JM. Severe Actinobacillus ureae meningitis in an immunocompromsed patient: Report of one case and review of the literature. Scand J Infect Dis. 2007. 39:1076–1079.

3. Starkebaum GA, Plorde JJ. Pasteurella pneumonia: report of a case and review of the literature. J Clin Microbiol. 1977. 5:332–335.

4. Maritz FJ, Franco MM, Swart WH. Pasteurella ureae septicaemia. A case report. S Afr Med. J. 1981. 59:53–54.
5. Yoshizaki E, Kamiki T, Sakazaki R, Tamura K. A case of bronchopneumonia possibly caused by Pasteurella ureae (author's transl). Kansenshogaku Zasshi. 1981. 55:534–536.
6. Pérez JA, de la Iglesia A, de la Iglesia M, Menchero A. Pneumonia caused by Actinobacillus ureae. Enferm Infecc Microbiol Clin. 2000. 18:296–297.
7. Gatti F, Seynhaeve V, Weaver R. 1st description of a case of human septicemia due to Pasteurella ureae. Ann Soc Belges Med Trop Parasitol Mycol. 1968. 48:463–468.
8. Barardi L, Bourdain J, Chatelain R, Riou J. Diagnostic bacterioligque de Pasteurella ureae: a propos d'un cas de septicemia humaine. Med et Maladies Infect. 1984. 14:36–40.
9. Bogaerts J, Lepage P, Kestelyn P, Vandepitte J. Neonatal conjunctivitis caused by Pasteurella ureae. Eur J Clin Microbiol. 1985. 4:427–428.
10. Bigel ML, Berardi-Grassias LD, Furioli J. Isolation of Actinobacillus urea (Pasteurella ureae) from a patient with otitis media. Eur J Clin Microbiol Infect Dis. 1988. 7:206–207.

11. Noble RC, Marek BJ, Overman SB. Spontaneous bacterial peritonitis caused by Pasteurella ureae. J Clin Microbiol. 1989. 27:375.
12. Yamamoto K, Ikeda U, Ogawa C, Fukazawa H, Eto M, Shimada K. Pasteurella ureae endocarditis. Intern Med. 1993. 32:872–874.

13. Vay C, Rodríguez C, Sadorin R, Vujacich P, Famiglietti A. Actinobacillus ureae isolated from a patient with chronic bronchitis. Enferm Infecc Microbiol Clin. 1995. 13:569–570.
14. Avlami A, Papalambrou C, Tzivra M, Dounis E, Kordossis T. Bone marrow infection caused by Actinobacillus ureae in a rheumatoid arthritis patient. J Infect. 1997. 35:298–299.

15. Kaur PP, Derk CT, Chatterji M, Dehoratius RJ. Septic arthritis caused by Actinobacillus ureae in a patient with rheumatoid arthritis receiving anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2004. 31:1663–1665.
16. Whitelaw AC, Shankland IM, Elisha BG. Use of 16S rRNA sequencing for identification of Actinobacillus ureae isolated from a cerebrospinal fluid sample. J Clin Microbiol. 2002. 40:666–668.

17. Mynter H. Acute pyositis. Buffalo Med Surg J. 1881. 21:202–210.
18. Taiwo B. Psoas abscess: a primer for the internist. South Med J. 2001. 94:2–5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download