Abstract
Gemellae is a gram positive cocci that forms part of the oropharyngeal microflora in humans and is anaerobic to aerotolerant. Unlike the other members of the same genus, G. morbillorum rarely causes human infections. Recently, we experienced a case of tubo-ovarian abscess caused by G. morbillorum which was initially suspected to be actinomycosis associated with intrauterine device. This is the first case in the world on tubo-ovarian abscess with G. morbillorum as the culprit.
Gemella morbillorum is a gram positive organism with coccoid morphology that is anaerobic to aerotolerant. It was classified as Streptococcus morbillorum in 1917 and bore its name until 1988 when DNA filter hybridization revealed the resemblance between this strain and Gemella haemolysan (1). G. morbillorum forms part of the oropharyngeal microflora in humans. An infection caused by G. morbillorum is unusual. A review of literature revealed that there have been several human infections caused by this organism such as endocarditis, phlebitis, septic arthritis, meningitis, spondylodiscitis in a patient with a renal graft, and bacteremia in children (2-5). However G. morbillorum infection has never been reported to cause gynecological diseases.
Recently, we experienced a case of G. morbillorum infection in tubo-ovarian abscess that was initially suspected to be a case of actinomycosis associated with an intrauterine device.
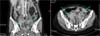
A 54-year-old woman (2-0-2-2) with an intrauterine device implanted about 10 years ago was admitted to the gastro-intestinal department with fever of two weeks' duration, lower abdominal pain, and absence of stool passage for 20 days. On the abdomino-pelvic computed tomography (CT), a right adnexal mass measuring 9.0×4.0×5.0 cm was observed accompanied by a left adnexal mass (5.6×6.7×6.4cm)(Fig. 1). An abnormal fistula was noted between the sigmoid colon and the right adnexal mass. The bilateral adnexal masses were considered to be abscess caused by Actinomycetes. The patient was transferred to the department of obstetrics and gynecology.
On admission, the patient was acutely ill-looking with vital signs as follows: blood pressure,110/70 mmHg; pulse rate, 76 times/min; body temperature, 37.8℃. There was tenderness on the lower abdomen and both adnexal areas with rebound tenderness. Cervical motion tenderness was also detected.
The laboratory tests results were as follows: hemoglobin, 11.0 g/dL; WBC, 18,400/mm3 (89.0% neutrophils, 8% lymphocytes); platelets, 448,000/mm3; Blood urea nitrogen(BUN), 4.9 mg/dL; creatinine, 0.7 mg/dL; total protein, 6.8 g/dL; albumin, 2.8 g/dL; aspartate aminotransferase, 34 IU/L; alanine aminotransferase, 22 IU/L; total bilirubin 0.6 mg/dL; and C-reactive protein 18.69 mg/dL. The chest posterior-anterior(PA) and simple abdomen X-ray radiographs were unremarkable. PAP smear was negative for malignancy.
On the third hospital day, after bowel preparation and right double-J catheter insertion, the patient underwent total abdominal hysterectomy, bilateral adnexectomy, and pelvic adhesiolysis. Contrary to the abdomino-pelvic CT results, severe inflammation and adhesions without fistula and bowel obstruction were identified during surgery. After the operation, pathologic reports confirmed the diagnosis of tubo-ovarian abscess with bilateral pelvic adhesion. Two days after surgery, fever subsided and three days afterwards, she began to defecate. G. morbillorum was grown from pus culture. The patient was given intravenous ampicillin/sulbactam and her general condition improved. She was discharged on the sixteenth day after surgery.
G. morbillorum was discovered in 1917 by Tunniciliff. Since it is an anaerobe, this bacteria was initially classified as the genus Peptostreptococcus which was then reclassified into the genus Streptococcus after its major metabolic product was found to be lactic acid (1). However, it was renamed once more in 1988 when DNA-DNA filter hybridization, guanine and cytosine content analysis, and 16S rRNA and oligonucleotide cataloguing revealed its resemblance to G. haemolysan (6). The 4 species of Gemella that have been implicated as human pathogens are G. morbillorum, G. haemolysans, G. bergeriae, and G. sanguinis.
Gemellae is anaerobic to aerotolerant and gram positive organism with a coccoid morphology; it forms part of the oropharyngeal microflora in humans. Unlike other members of the genus, human infections caused by G. morbillorum are rare. It was revealed 15.5% isolation of S. morbillorum from vaginal exudates in Espana study and pregnant women kept S. morbillorum about 2%(7,8).
A review of the literature revealed that there have been several human infections caused by this organism such as endocarditis, phlebitis, meningitis, septic arthritis, as well as 2 cases of bacteremia in children (9-11). In both pediatric cases, association was found between G. morbillorum and septic shock syndrome, which was fatal in 1 case; the organism is presumed to have seeded into the bloodstream from the oral flora following intubation in the first case, whereas bacteremia appears to have been a complication of maxillary sinusitis in the second case. These cases suggest that the infection caused by G. morbillorum is an opportunistic infection that occurs in immuno-suppressed patients. We found two case reports that were published in Korea; one case was bacteremia and the other case was liver abscess caused by G. morbillorum (12, 13). Predisposing factors for infection by G. morbillorum include poor dental hygiene, dental procedure, colon disease, and gastrointestinal diagnostic procedure (9). The portal of entry of the organism in our patient was not obvious because of the absence of any infection foci. This is the first case report in the world on tubo-ovarian abscess caused by G. morbillorum in an immunocompetent host. Virulence factors have not been extensively studied, but exopolysaccharide production has been implicated in both endocarditis and genital tract infections (3).
Diagnosis is difficult because cells are easily decolorized during Gram staining and may, therefore, appear to be Gram variable or even Gram negative. It is likely that morphological polymorphism is responsible for the misidentification of Gemella spp. which results in few reported cases of Gemella infection (10). All Gemella species have typical biochemical profile that includes positive leucine aminopeptidase and pyrrolidonyl arylamidase reaction, negative reactions for catalase, esculin, arginine, urease, and hippurate, and growth in 6.5% sodium chloride. A positive pyrrolidonyl arylamidase will rule out Streptococcus species. More recently, 16S rRNA gene sequencing has been recommended for the identification of Gemella and Gemella-like organisms(6, 11, 14). Gemella should be considered when slow-growing, catalase-negative, Gram positive cocci are seen in samples.
Antibiotic treatment against G. morbillorum associated infections does not seem to be difficult. Almost all cases have been successfully cured by antibiotics therapy. Bacteriological cure was achieved with a combination of penicillin and an aminoglycoside in endocarditis. In patients that were either allergic or resistant to penicillin, vancomycin or a combination erythromycin and rifampin has been effective (9, 15-18). However, recent data suggest emerging penicillin and macrolide resistance. Linezolid is used when it is resistant to penicillin, aminoglycoside, and vancomycin.
In our case, a 54-year-old woman, despite having intrauterine device, was not thought to be in an immuno-suppressed state. Furthermore, we initially considered this case to be a tubo-ovarian abscess associated with intrauterine device caused by Actinomycetes, but G. morbillorum was responsible for this infection. G. morbillorum does not commonly cause tubo-ovarian abscesses. To the best of our knowledge, this is the first case report on tubo-ovarian abscess caused by G. morbillorum.
Figures and Tables
References
1. Kilpper-Balz R, Scleifer KH. Transfer of Streptococcus morbillorum to the genus Gemella as Gemella morbillorum comb. nov. Int J Syst Bacteriol. 1988. 38:442–443.

2. Calopa M, Rubio F, Aguilar M, Peres J. Giant basilar aneurysm in the course of subacute bacterial endocarditis. Stroke. 1990. 21:1625–1627.

3. Omran Y, Wood CA. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993. 16:131–134.

4. Debast SB, Koot R, Meis JF. Infections caused by Gemella morbillorum. Lancet. 1993. 342:560.
5. Eisenberger U, Brunkhorst R, Perharic L, Petersen R, Kliem V. Gemella morbillorum-spondylodiscitis in patient with a renal graft. Nephrol Dial Transplant. 1998. 13:1565–1567.
6. Woo PC, Lau SK, Fung AM, Chiu SK, Yung RW, Yuen KY. Gemella bacteraemia characterised by 16S ribosomal RNA gene sequencing. J Clin Pathol. 2003. 56:690–693.
7. Rabe LK, Winterscheid KK, Hillier SL. Association of viridans group streptococci from pregnant women with bacterial vaginosis and upper genital tract infection. J Clin Microbiol. 1998. 26:1156–1160.

8. Egido JM, Maestre JR, Peña Izquierdo MY. The isolation of Streptococcus morbillorum from vaginal exudates. Rev Soc Bras Med Trop. 1995. 28:117–122.
9. Zakir RM, Al-Dehneh A, Dabu L, Kapila R, Saric M. Mitral bioprosthetic valve endocarditis caused by an unusual microorganism, Gemella morbillorum, in an intravenous drug user. J Clin Microbiol. 2004. 42:4893–4896.

10. La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol. 1998. 36:866–871.

11. Valipour A, Koller H, Setinek U, Burghuber OC. Pleural empyema associated with Gemella morbillorum: report of a case and review of the literature. Scand J Infect Dis. 2005. 37:378–381.

12. Kim MJ, Park YS, Kim SY, Seo YH, Chang US, Cho YK. A case of bacteremia due to Gemella morbillorum. Infect Chemother. 2005. 37:226–229.
13. Nam HJ, Yoon SJ, John BM, Jung SH, Kim A, Ko BS, Yang HW, Hwang KY, Lee JY, Kim SH, Kim DJ, Kim NY, Lim SH. Liver abscess caused by Gemella morbillorum. Korean J Gastroenterol. 2005. 46:56–59.
14. Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis. 1996. 22:1084–1086.
15. Lai CC, Wu CH, Chen JT, Hsueh PR. Peritoneal dialysis-related peritonitis caused by Gemella morbillorum in a patient with systemic lupus erythematosus receiving steroid therapy. J Microbiol Immunol Infect. 2008. 41:272–274.
16. Famularo G, De Simone C, Minisola G, Nicotra GC. Pneumonia and sepsis caused by Gemella morbillorum: an unusual association. Intern Med. 2006. 45:1253–1254.

17. Bachmeyer C, Landgraf N, Daumas L. Soft tissue infection caused by Gemella morbillorum in two intravenous drug users. J Am Acad Dermatol. 2005. 52:704–705.

18. Zheng M, Ng OT, Teo BW. Aortic and mitral valve endocarditis caused by Gemella morbillorum in a haemodialysis patient. Singapore Med J. 2008. 49:e385–e387.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download