Abstract
Burkholderia cepacia is an important pathogen of humans in both immunocompromised or hospitalized patients. This aerobic gram-negative rod causes various clinically significant infections such as pneumonia, bacteremia, urinary tract infection, peritonitis, endophthalmitis, and keratitis. This uncommon pathogen is mainly associated with cystic fibrosis, dialysis, and immunocompromised states. We observed a 73-year-old woman without any underlying diseases, who had both lower leg pain and fever. She was later found to have spondylitis with epidural abscess at L5-S1. Culture of the epidural abscess revealed the growth of B. cepacia. To the best of our knowledge, this is the first case of pyogenic spondylitis caused by a rare infection of B. cepacia to be reported in Korea
Burkholderia cepacia is a gram-negative rod that can be found in soil, water, and other environmental sources (1-3). B. cepacia is recognized as uncommon pathogen causing serious infection particularly in patient with cystic fibrosis and immunodeficiency such as chronic granulomatous disease (3). Most cases of B. cepacia infections have been bacteremia with septic shock, nosocomial pneumonia, septic arthritis, and soft tissue infection (1-6). Spondylitis is a rare manifestation of B. cepacia infection. We report here on a case of spondylitis by B. cepacia in a healthy adult.
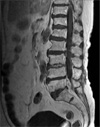
A 73-year-old woman was admitted to the emergency room with pain at both lower legs, fever and chilling that had aggravated over period of one week before admission. She has been treated with intramuscular stimulation therapy and physiotherapy due to a backache that had worsened since she slipped down on an icy road three weeks before admission. Four years prior to this admission, she received partial laminectomy of L5. She had a past medical history of hysterectomy, senile cataract, and hypertension, having anti-hypertensive drugs at the time of admission. On physical examination, fever or local tenderness in the paravertebral area were not observed at the first day of hospitalization. Although no febrile sensation was accompanied on the day of admission, her body temperature rose up to 39.3℃ on the next day. Blood and urine cultures were taken. Hematologic investigation revealed a hemoglobin level of 10.2 g/dL, a platelet count of 377×103/µL, and white cell count of 9.56×103/µL (76.9% segmented neutrophils). Erythrocyte sedimentation rate was 68 mm/hour, and C-reactive protein (CRP) was 11.6 mg/dL. Plain roentgenograms of the spine showed compression fracture of the vertebral bodies of the T12 and L1, and partial laminectomy state in L5. Magnetic resonance imaging confirmed epidural abscess, discitis, and edema in adjacent L5 to S1 vertebra (Fig. 1). Initially, she was treated empirically with vancomycin, ceftriaxone, and gentamicin. The discectomy with postero-lateral fixation was performed at the third day of hospitalization, and pus culture was taken during surgery. Pus-like material obstained during the operation was cultured both aerobically and anaerobically. After 24 hours of incubation, pale yellow, irregular, smooth, and non-hemolytic colonies were evident in the plates. On Gram stain, the isolates were found to be gram-negative rods. The initial identification of the isolate was Chryseobacterium meningosepticum on the seventh postoperative day. The identification and antibiotic susceptibility tests of the bacteria were performed using the MicroScan WalkAway (DADE BEHRING INC., West Sacramento, CA, US). While the isolate was resistant to all kinds of penicillin, cephalosporin, beta-lactam/beta-lactamase inhibitors, aminoglycosides, and trimethoprim/sulfamethoxazole, it was sensitive to moxifloxacin, levofloxacin, and imipenem. According to antibiotic susceptibility results, the therapy was changed to levofloxacin 750 mg per day intravenously. Additionally 16S-rRNA sequencing was performed since there has been no report of C. meningosepticum-associated spondylitis in immunocompetent hosts. Finally B. cepacia was confirmed by sequencing of 16S-rRNA. On the fifteenth postoperative day, a second operation (anterior corpectomy with bone graft) was performed. Her clinical conditions improved rapidly after levofloxacin therapy and second operation. Three weeks after the second operation, she was discharged from the hospital with oral levofloxacin. The CRP was below 0.5 mg/dL after total six weeks of levofloxacin therapy. The final examination performed three months after discharge showed that the patient was in good health.
B. cepacia, formerly Pseudomonas cepacia was first described as a phytopathogen of onion rot in the 1950s (1). B. cepacia is a non-fermenting, motile, aerobic Gram-negative bacillus that proliferates under conditions of minimal nutrition, and can survive even in the presence of certain disinfectants and antiseptic solutions (1-3).
This bacterium is ubiquitous in soil, water, and plants (1, 3, 5). The environment also serves as a source of acquiring this pathogen (1). In the hospital setting, this pathogen has been isolated in water taps, distilled water, nebulizers, water baths, dialysis fluids and machines, contaminant disinfectants, solutions and intravenous fluids, catheters, blood gas analyzers, thermometers, and ventilator temperature sensors (1, 2, 4). Although an immunocompetent person may develop necrotizing pneumonia or liver abscess (7, 8), isolation of this organism should alert the clinician to either an underlying immunodeficiency- or health care-associated infection (3).
There are several predisposing factors including indwelling catheter, ventilator care, and cystic fibrosis in context of nosocomial infections (1). There are case reports about septic arthritis, cervical necrotizing fasciitis, sepsis, and infected vertebroplasty caused by B. cepacia (1-6). Approximately 90% of the 269 strains of B. cepacia by Sader and Jones were resistant to polymyxin B, 85% to tetracycline, tobramycin, and gentamicin, 75% to ticarcillin/clavulanate, 70% to amikacin, 50% to aztreonam, 35% to ceftriaxone, 30% to cefepime, ciprofloxacin, 20% to imipenem, meropenem, gatifloxacin, and levofloxacin, 15% to piperacillin/tazobactam, 10% to piperacillin, trimethoprim/sulfamethoxazole, and ceftazidime (9). This implies there must be some limitation on the therapeutic options. Antimicrobial agents that are effective against B. cepacia include meropenem, chloramphenicol, minocylcline, third-generation cephalosporins, and fluoroquinolones (1, 2). Combinations that have shown synergy in vitro include ciprofloxacin and piperacillin, tobramycin and piperacillin, trimethoprim/sulfamethoxazole and ceftazidime, ciprofloxacin and carbapenem, ceftazidime and amikacin, or ceftazidime and ciprofloxacin (1). For empirical therapy of B. cepacia infections, ceftazidime and/or trimethoprim/sulfamethoxazole should be the drug of choice (1).
B. cepacia is ubiquitous in soil and water. The environment also serves as a source of this pathogen (10). A common route of transmission is by either nosocomial or social contact in cases of cystic fibrosis (7). Social transmission in a non-cystic fibrosis individual has not been yet described (7). Nosocomial infections included bacteremia, meningitis, endocarditis, pneumonia, surgical wound infection, and urinary tract infection (10). In a hospital setting, contaminated sink basins, taps, and other medical devices are known as potential reservoirs for infection. Community acquired bactermia is rare, but there has been two reported cases of infective endocarditis 1. A case of community acquired pneumonia in an immunocompetent adult was reported and the most likely source of this case seems to be from a non-hospital environment such as an agricultural product.
It is suspected that infection probably initiated from an external wound which was unnoticed by the patient on the fall on the icy road or from the intramuscular stimulation therapy procedure which may have played a role as the routes of invasion. Although, neither is confirmable, the source of B. cepacia may be most likely hospital environment considering the pattern of antimicrobial susceptibilities.
To the best of our knowledge, this is the first case of pyogenic spondylitis caused by B. cepacia in Korea. In our opinion, B. cepacia should be considered as a causative, uncommon pathogen in future cases of pyogenic spondylitis. Our reported case demonstrates that the definitive diagnosis of spondylitis should be made based upon bacteriologic examination, and the result of culture studies on the infectious tissue is essential for the appropriate treatment against the uncommon pathogen or multi-drug resistant organisms.
Figures and Tables
References
1. Huang CH, Jang TN, Liu CY, Fung CP, Yu KW, Wong WW. Characteristics of patients with Burkholderia cepaciacia bacteremia. J Microbiol Immunol Infect. 2001. 34:215–219.
2. Marioni G, Rinaldi R, Ottaviano G, Marchese-Ragona R, Savastano M, Staffieri A. Cervical necrotizing fasciitis: a novel clinical presentation of Burkholderia cepacia infection. J Infect. 2006. 53:e219–e222.
3. Held MR, Begier EM, Beardsley DS, Browne FA, Martinello RA, Baltimore RS, McDonald LC, Jensen B, Hadler JL, Dembry LM. Life-threatening sepsis caused by Burkholderia cepacia from contaminated intravenous flush solutions prepared by a compounding pharmacy in another state. Pediatrics. 2006. 118:e212–e215.
4. Ramsey AH, Skonieczny P, Coolidge DT, Kurzynski TA, Proctor ME, Davis JP. Burkholderia cepacia lower respiratory tract infection associated with exposure to a respiratory therapist. Infect Control Hosp Epidemiol. 2001. 22:423–426.

5. Alfonso Olmos M, Silva González A, Duart Clemente J, Villas Tomé C. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure report of a case and a review of the literature. Spine. 2006. 31:E770–E773.
6. Miki RA, Rubin LE, Kirk J, Dodds SD. Spontaneous septic arthritis caused by Burkholderia cepacia. Iowa Orthop J. 2006. 26:147–150.
7. Jaramillo-de la Torre JJ, Bohinski RJ, Kuntz C 4th. Vertebral osteomyelitis. Neurosurg Clin N Am. 2006. 17:339–351.

8. Ledson MJ, Gallagher MJ, Walshaw MJ. Chronic Burkholderia cepacia bronchiectasis in a non-cystic fibrosis individual. Thorax. 1998. 53:430–432.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download