INTRODUCTION
The primary and most successful approach for the treatment of cancer is still surgery, even though radiotherapy and chemotherapy techniques are widely used. However, most human surgeries are performed without any image guidance meaning that the practice depends completely on the visual ability of the surgeon. As a result, one of the fundamental limitations of cancer surgery, and in particular of minimally invasive surgery, is that the tumor tissue needs to be visually distinguished from the healthy tissue because most clinical imaging modalities cannot provide the desired target images in real-time.123456
Additionally, biomedical imaging modalities including ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) and single photon emission computed tomography (SPECT) are typically used to noninvasively access the disease tissue prior to the surgical procedures, but they are not available in the operating room. Furthermore, the ionizing radiation associated with these techniques limits the overall scope of their real-time translation.7891011
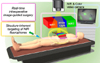
In response to this unmet, clinical need, optical imaging provides real-time visualization of the surgical field especially when combined with near-infrared (NIR) fluorophores, allowing intraoperative image-guided surgery.121314151617 Imaging in the NIR window (650-900 nm), known as the “therapeutic window”, has shown clinical potential by offering low absorbance and scattering in tissues as a black background while providing maximum depth of light penetration. The advantages of NIR fluorescence have been recognized and many types of fluorescence image-guided surgery systems are continuously developing in both the preclinical and clinical phases.1819 Thus, with the right combination of an NIR fluorescence imaging system and targeted NIR fluorophores, the desired target tissues can be imaged to support real-time fluorescence guidance with unchanging field of view during surgery (Fig. 1).202122232425
To provide the targeted visualization for more precise surgery, NIR contrast agent development and clinical translation are necessary. However, the development of ideal NIR fluorophores has been a longstanding problem in the field of fluorescence image-guided surgery.2627282930 It is important to note that the visualization of the target tissue mainly depends on the efficacy of fluorescent agents, resulting from the difference in the amount of molecular uptake into the target and neighboring normal tissues. However, it is difficult to achieve a high quality, targeted image due to the limited number of biomarkers and targeting ligands. Currently, there are only two FDA approved NIR fluorophores, indocyanine green (ICG) and methylene blue (MB). Both ICG and MB are blood pool agents that have no inherently specificity for tumor or normal tissues, and thus are not ideal fluorophores for fluorescence image-guided surgery.313233343536373839 Therefore, in a clinical discipline, the development of NIR fluorophores for target-specific imaging is an unmet need for early phase diagnosis with accurate targeting.
In this review, we focus on the latest achievements in structure-inherent targeting by developing NIR fluorophores for fluorescence image-guided surgery resulting in a high potential to shift the clinical paradigms.
STRUCTURE-INHERENT TARGETING OF NIR FLUOROPHORES
NIR fluorescence imaging techniques can provide target-specific imaging with a high degree sensitivity for target detection because of the ultralow background autofluorescence prior to excitation of the target tissue. The improved detection sensitivity makes it possible for the application to fluorescence-guided surgery. However, a fundamental problem in fluorescence-guided surgery is the development of well-defined fluorophores that target specific normal or diseased tissues. For the targeted fluorescence imaging, the conventional method for preparing targeted imaging agents requires covalent conjugation of separate targeting moieties and fluorescent materials. In this review, we summarize research showing that it is possible to create NIR fluorophores with different tissue specificities driven by their inherent chemical structures (Table 1 and Fig. 2). A single fluorophore could be designed to achieve selective targeting with high efficiency in differentiating specific tissues through modifying the physicochemical properties and incorporating recognition elements into the fluorophore domains. Selective modifications control the pharmacophore to binding sites of the target tissues directed by structure-inherent chemical recognition. Thus, the single compact fluorophore performs both targeting and imaging, simultaneously. This strategy is the paradigm shift in a role of fluorophores from traditional method used for covalent conjugation of targeting ligands and fluorescence imaging agents.
1. Parathyroid and thyroid glands targeting
The endocrine glands are tissues of substantial clinical importance, yet they are difficult to locate during surgery. In particular, a partial or complete resection of the thyroid gland requires accurate identification and preservation of the parathyroid glands. Complications can occur when all functional parathyroid glands are inadvertently damaged or removed during thyroidectomies, or when they are incompletely removed during parathyroidectomies. Damage to these tiny organs, which secrete hormones that regulate blood calcium levels, can have deleterious, lifelong effects on a person's health by causing conditions such as hypocalcemia.
The identification of normal parathyroid glands with the naked eye is still challenging in high-risk procedures. Not only are these tissues small, but their location also varies widely from person to person. Moreover, surgical biopsy of the parathyroid for identification can lead to devascularization and destruction of its function. Consequently, surgeons must rely on visual inspection to identify the different tissues, which can be subjective and inconclusive.
Recently, Hyun et al. developed two NIR fluorophores that are efficiently taken up by the thyroid and parathyroid glands after intravenous injection.40 They tested the NIR fluorophores in rats and pigs and showed that they could be used to distinguish the thyroid and parathyroid glands from surrounding tissue and from each other. The mechanism of uptake and retention of T700-F (thyroid targeting NIR fluorophore) and T800-F (parathyroid targeting NIR fluorophore) in specific cell types of the thyroid and parathyroid glands is still unknown. However, it appears that site-specific halogenation of the polymethine core is crucial for targeting, and it is possible that iodine-processing cellular machinery (such as transporters and enzymes) may be mistaking these molecules as endogenous substrates.
This technique has shown excellent potential application for increased precision in head and neck surgeries and thus fewer surgical accidents and lower mortality. This approach also provides design considerations for developing disease-specific contrast agents with a strategy of ‘structure-inherent targeting’.
2. Lymph nodes targeting
The sentinel lymph node (SLN) is the first lymph node receiving lymphatic drainage from a primary tumor. SLN biopsy is essential for staging many cancers and avoiding unnecessary LN dissection, which may cause adverse effects such as lymphedema. However, there is currently no technology available to surgeons to help them identify all LNs within a basin quickly and with high sensitivity.
Previous reports demonstrated that the use of invisible NIR light has several advantages for SLN biopsy, including relatively high penetration (up to 5-8 mm) into living tissue. When compared to blue dye MB, invisible NIR light has a relatively high signal-to-background ratio (SBR) due to low absorption, scattering, and tissue autofluorescence. Because of these advantages, Josephson et al. employed Cy5.5-grafted polymers (PGC) to demonstrate NIR imaging of pan lymph nodes (PLN), and the Vahrmeijer group demonstrated preclinical evidence by using the premixing of ICG with human serum albumin, resulting in better retention in the SLN.41424344454647 However, none of these studies used the NIR fluorophores for ‘structure-inherent targeting’ of LNs, because Cy5.5 and the currently available clinical NIR fluorophores, MB and ICG, cannot visualize PLNs after intravenous injection.
Recently, Ashitate et al. reported that engineered novel NIR fluorophores have fluorescence emission maxima in two distinct ranges; one NIR color for visualization of PLNs and the other for mapping SLN in the same subject.48 They achieved the successful mapping of PLNs and SLN by using the single NIR fluorophore different from previous studies by using nanoparticles and polymers. They also emphasized that the proper statistical assessment of SLN biopsies, which includes sensitivity, specificity, positive predictive value, negative predictive value, and accuracy, is dependent on finding all LNs within a basin. Thus, this technique will permit improved quantitative evaluation of SLN biopsy for cancers other than breast cancer and melanoma.
3. Nerve tissue targeting
Nerve damage during surgery results in significant morbidity and mortality. However, nerves are still identified via gross appearance and anatomical location, without any intraoperative image guidance. To minimize high morbidity and mortality from nerve injury, intraoperative visual identification during complex surgeries is of paramount importance.
Although only 3 main classes of nerve-specific molecules have been reported to date, including stilbene derivatives, distyrylbenzene (DSB) fluorophores, and styryl pyridinium (FM) fluorophores, none of these compounds were optimal because of high adsorption and scattering in tissues from the ultraviolet and visible wavelengths required.49 In addition, Tsien and colleagues reported a significant improvement in nerve-specific targeting and imaging, where an engineered peptide FAM-NP41 was used to label in vivo animal nerves and resected human recurrent laryngeal nerves with relatively high affinity.50
Unlike this approach using macromolecules, Park et al. recently investigated an ideal, nerve-specific NIR fluorophore named Oxazine 4 for image-guided surgery.51 Based on their criteria to design the nerve-specific small molecule, it should have logD at pH 7.4 between 0.5 to 3 and a molecular weight <500 Da to maximize blood-nerve-barrier (BNB) penetration, excitation and emission wave-lengths in the NIR window, and prolonged retention in the nerve tissue.
Importantly, Oxazine 4 does not stain the central nervous system, staining only the peripheral nervous system, and did not show apparent impairment of motor function and sensory integrity during a set time period. Therefore, Oxazine 4 will be a good reference to develop the optimal nerve-specific NIR fluorophore providing real-time image-guidance to surgeons about nerve locations during surgery.
4. Bone tissue targeting
All targeted NIR fluorophores described to date for bone imaging require covalent conjugation of a targeting domain (i.e., divalent cation chelating agents such as calcein and tetracycline, bone targeting drugs such as pamidronate, etc.) to a NIR fluorophore.52535455
For the structure-inherent targeting of bone tissue, Hyun et al. recently reported a new strategy based on the incorporation of targeting moieties into the nonresonant structures of polymethine indocyanines emitting at NIR wavelength range.56 Using the known affinity of phosphonates for bone minerals as a model system, they developed two families of bifunctional molecules that target bone without the need for a traditional bisphosphonate.
Additionally, longitudinal NIR imaging studies in mice demonstrated that phosphonated NIR fluorophores remain stable in bone tissue for over 5 weeks, and histological analysis demonstrated incorporation into the bone matrix without interfering with normal bone deposition. Therefore, the bifunctional NIR fluorophore could be used to attach drugs that modulate osteoblasts or osteoclasts, creating trifunctional theranostic agents. They emphasized that non-hormonal phosphonates increase bone strength, and repeated injections of this theranostic agent will improve bone density by preventing the loss of minerals. Taken together, this approach will be a new strategy for bone-specific imaging by using the targeted NIR fluorophores.
5. Cartilage tissue targeting
The major techniques for visualization of cartilage tissue are MRI and CT, but neither technique provides optimum image quality. A new method recently reported by Hyun et al. is based on fluorescence imaging agents that can be detected in the NIR wavelength range.57 Injection of the cartilage-specific NIR fluorophores allows high-quality visualization of cartilage tissue in real-time. The most important advantage of using NIR fluorophores for cartilage imaging is the integrated method of performing ‘structure-inherent targeting’ (i.e. molecular targeting and fluorescent imaging) using a single small molecule. The high affinity of cationic NIR fluorophores for cartilage provides high-quality tissue visualization, ultralow background signal, and low injected doses.
Although it is not clear yet what the actual binding target of the NIR fluorophore is, they assume that the highly negatively charged components of the cartilage tissue are the targets that are recognized and specifically bound by the positively charged fluorophores. Typically, it is thought that cationic materials including nanoparticles and polymers show high nonspecific binding in the body. The cationic NIR fluorophores, however, are engineered by modulating the nonresonant side chains of the polymethine core; they can be systematically modified to tune physicochemical properties such as hydrophobicity, polarity, and electron-resonance properties without affecting binding affinity. In addition, the new NIR fluorophores are highly stable and do not degrade in the body, and a strong cartilage signal was obtained 1-4 hours after the injection of a single dose. Moreover, the cartilage-specific NIR fluorophores make it possible to detect cartilage and bone tissue simultaneously in real-time by combining with bone-specific NIR fluorophores.
Importantly, NIR fluorophores provide high spatial resolution in real-time to distinguish the boundary and thickness of cartilage tissues from the adjacent bones, where other contrast agents with nanoparticle formulations have limited penetration and delineation. Thus, the new cartilage-specific NIR fluorophores have many beneficial applications in terms of cartilage degeneration, new drug design, and image-guided surgery.
6. Adrenal, pituitary and salivary glands targeting
During endocrine surgeries, surgeons depend on their naked eye and experience during often-lengthy procedures to avoid sensitive glands and tissues. The important task of avoiding sensitive tissues is difficult as the small glands are obscured by blood and surrounding tissues. Surgeons need to visualize these tissues during cancer surgery to improve the surgical success rate and advance patient prognosis.
Among the endocrine glands, the adrenal glands located above the kidney produce essential and nonessential hormones that primarily control the body's metabolism, blood pressure, and stress response. The pituitary gland is also responsible for controlling the hormonal release of signaling chemicals throughout the body. In addition, imaging of exocrine salivary glands is useful in identifying their masses and also in distinguishing them from the masses/pathologies of adjacent cervical spaces.
Significant advancements have been reported in the development of NIR fluorophores that target tissues and help surgeons avoid these hormonal glands without changing the surgical field of view in real-time. This class of NIR fluorophores has shown excellent promise in the field of NIR fluorescence-guided surgery for targeting of tumor- and/or normal tissues.
Recently, Owens et al. systematically developed various NIR fluorophores that focused on the effects on biodistribution based on modulating electronic influencing moieties from donating to withdrawing moieties at both the heterocyclic site and through meso-substitution of pentamethine cyanine fluorophores.585960 The selective modifications controlled innate biodistribution routes through ‘structure-inherent targeting’ resulting in efficient imaging of the adrenal glands, pituitary gland and salivary glands. These tissues are important for the regulation of human hormone levels and should be carefully avoided during surgical resection in surrounding areas. Thus, these native-tissue specific NIR fluorophores will help surgeons by providing a powerful guidance tool for intraoperative NIR imaging in real-time.
7. Pancreas targeting
Recent technical advances in abdominal surgery have assisted in making complex surgical procedures safer and shorter. However, pancreas-related complications after gastrectomy with radical lymphadenectomy including peripancreatic lymph nodes resection, splenectomy, adrenalectomy, and nephrectomy are still high. Intraoperative pancreatic injuries are typically caused by the proximity of the pancreas to surrounding organs and the difficulty in distinguishing the edge of the pancreas from surrounding fat tissue, especially in obese patients.
Intraoperative identification of the pancreas with high sensitivity in real-time could help avoid such injuries. Previously, Winer et al. reported that NIR fluorescent light could be used to identify both the pancreas and insulinoma by employing the clinically available NIR fluorophore MB.61 Although MB provided contrast between the pancreas and surrounding organs, it is not an ideal NIR contrast agent because of low fluorescence intensity, non-specific uptake, and short retention time in the pancreas.
More recently, Wada et al. reported pancreas-specific NIR fluorophore T700-F that showed significantly high uptake in the pancreas and low background uptake in the neighboring tissue. Furthermore, its retention period in the pancreas was over 8 h, making this fluorophore potentially useful in complex abdominal surgeries including laparoscopic operations.62 Although the mechanism of specific pancreas uptake and retention of T700-F is still unknown, it shows that this approach could be used to identify the pancreas tissue and the novel NIR fluorophore could outperform MB. Thus, it will be a great candidate for real-time intraoperative pancreas imaging during complex abdominal surgeries.
CONCLUSION AND FUTURE PERSPECTIVES
Real-time image-guided surgery is gaining attention in oncologic surgery because it has a high potential to improve clinical outcomes. Not only can this approach direct intraoperative image guidance for surgical margin assessments, the approach can help surgeons detect microscopic tumors or residual lesions that are unconsciously missed during surgery. If surgeons are able to find tumors completely or avoid neighboring normal tissues more easily then operating time could be shortened, resulting in reduced anesthesia time and its associated risks. The improved surgical system for a successful surgery lowers recurrences and complication rates so it is an excellent way to save on healthcare costs.
The current bottleneck preventing the potential benefits of fluorescence image-guided surgery is the lack of FDA approved tumor-, tissue-selective fluorescence imaging agents. Although successful preclinical studies of NIR fluorophores have recently been reported, none of them, except for ICG and MB, are yet available in clinics pending FDA approval. For clinical translation, the NIR fluorophores need to be designed by considering physicochemical and optical stability, selective targeting ability, reasonable pharmacological activity, efficient elimination from the body, and non-toxicity. Among these considerations, toxicity issue is a major hurdle for the FDA approval, because improper biodistribution and incomplete clearance can cause potential toxicity threats such as reproductive risks, immunotoxicity, neurotoxicity, acute/chronic toxicology, and genotoxicity through biological interactions in the body. Therefore, focusing on rapid biodistribution and complete clearance are essential for safety. That is, the target-specific NIR fluorophores should be designed for complete excretion from the body in a certain period time after achieving site-specific targeting during image-guided surgery. The in vivo fate of targeted NIR fluorophores is affected by various factors including hydrodynamic diameter, formulations, surface charge distribution, administration route, injection dose, protein binding, blood flow, and blood pH. Thus, retention and clearance of the injected NIR fluorophores in the target tissues/organs is based on the difference between distribution and elimination behaviors that plays a key role in the controlled accumulation period of these agents in the targets. Therefore, we believe that a fuller understanding of the molecular properties, tissue properties, biodistribution, and targeting mechanisms of these NIR fluorophores is the only way to overcome the barriers to their clinical use.
With continued progress, more preclinical trials will be reported and NIR imaging systems will become a more common technology in clinic. Furthermore, the innovative design and optimization of NIR fluorophores has the potential to lay a foundation in the development of other target-specific small molecule contrast agents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download