Abstract
Heptamethine cyanine dyes are categorized as a class of near infrared fluorescent (NIRF) dyes which have been discovered to have tumor targeting and accumulation capability. This unique feature of NIRF dye makes it a promising candidate for imaging, targeted therapy and also as a drug delivery vehicle for various types of cancers. The favored uptake of dyes only in cancer cells is facilitated by several factors which include organic anion-transporting polypeptides, high mitochondrial membrane potential and tumor hypoxia in cancer cells. Currently nanotechnology has opened possibilities for multimodal or multifunctional strategies for cancer treatment. Including heptamethine cyanine dyes in nanoparticle based delivery systems have generally improved its theranostic ability by several fold owing to the multiple functionalities and structural features of heptamethine dyes. For this reason, nanocomplexes with NIRF heptamethine cyanine dye probe are preferred over non-targeting dyes such as indo cyanine green (ICG). This review sums up current trends and progress in NIRF heptamethine cyanine dye, including dye properties, multifunctional imaging and therapeutic applications in cancer.
Near-infrared fluorescence (NIRF) imaging is an appealing method for cancer diagnosis at the early stage owing to its multi-detection capabilities and high sensitivity. Heptamethine cyanine dyes accumulate in tumor tissues and hence, have recently gained popularity for utilization in both imaging and therapy. Indocyanine green (ICG) is an optical imaging agent approved by the FDA and therefore the most widely used NIRF dye for imaging and therapeutic studies.12345678 Drawbacks of ICG include its inability to accumulate in tumors and inapplicability in tumor tracking for long-term theranosis. Newly synthesized analogues of heptamethine dyes, such as IR780, IR808, IR820, and MHI-148, have therefore been examined as alternatives for both imaging and therapeutic purposes (Fig. 1). Inorganic materials, such as gold, iron oxide nanoparticles, prussian blue, and graphene, as well as organic materials, such as polydopamine, have also been widely studied as optical or photothermal agents against various tumor types. The safety and efficacy of these materials is however yet to be proven as their accumulation in tumors is mainly dependent on enhanced permeability and retention (EPR) effects rather than active targeting, which leads to non-specific accumulation.910111213141516171819 Heptamethine probes display tumor eradicating action upon laser (808 nm) irradiation, which presents an opportunity in photothermal therapy (PTT), photodynamic therapy (PDT), and other combinatorial therapeutic methods. Furthermore, NIRF dyes that are loaded or conjugated to nanoparticles and anticancer agents participate in combination to the collegial treatment of cancer. The combined advantage of NIRF dyes for imaging and therapeutic function thus contributes to the effective diagnosis and treatment of target cancers. The intention of this review is to provide a suitable and brief update on the promising NIRF heptamethine cyanine dyes and multifunctional agents.
NIRF heptamethine dyes belong to a category of the ICG family that were recently discovered to have cancer targeting properties in combination with PTT and PDT.20 Analogues of heptamethine dyes include MHI-148, IR813, IR808, and IR780 (Fig. 2), which were initially utilized for the detection of cancers, e.g. detection of human kidney cancer using MHI-148 and IR783. They were found to be effective in detecting circulating blood and kidney cancer cells.21 Key advantages of heptamethine dyes, over other NIR dyes, are their abilities to accumulate in tumors,22 their stability, and their superior signal to noise ratios. Additionally, heptamethine dyes ensure minimal auto-fluorescence due to the high penetration depth in the NIR spectrum.2324
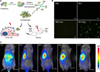
The unique features of heptamethine dyes include their capability for cancer accumulation and to remain there for extended periods, which imparts excellent signal to noise ratios. Heptamethine dyes primarily accumulate in cancer cells because of the cell's high mitochondrial membrane potential in comparison to normal cells.27 OATP1B3, a subtype of the organic anion transporting polypeptides (OATPs), is another factor responsible for the tumor specific uptake of heptamethine dyes.28 The uptake mechanism for the heptamethine dye analogue MHI-148 was indicated to be through the combined effect of OATPs and hypoxia-inducible factor 1α (HIF1α).29 MHI-148 dye uptake mechanisms in canine cancer cells and human prostate cancer cells (PCa) have also been explored (Fig. 3A). In addition, MHI-148 uptake by spontaneous canine testicular tumors was confirmed through Gallium complexation and positron emission tomography (PET). The capability of both IR783 and MHI-148 to accumulate in tumor tissues was also shown in kidney cancer xenograft mice and an ex vivo human kidney sample.21 In a more recent study, accumulation of MHI-148 was shown in patient-derived tumor xenograft (PDX) mice models and clinical gastric cancer specimens perfused with the dye (Fig. 3B, C).30 Heptamethine dye accumulation in the mitochondria of tumor cells has been shown using IR780 iodide and IR780 conjugated with nitrogen mustard (IR780NM). In vivo imaging showed that IR780NM retained its tumor-targeting property, suggesting that IR780 iodide is a good candidate for as a drug delivery agent for cancer-targeted imaging and therapy.253132
Heptamethine dye has excellent tumor-targeting properties, high quantum yield, and low autofluorescence in tissues, making them excellent tumor imaging probes.33 Colon cancer detection was achieved using NIR fluorescent nanoparticles containing IR783. Nanoparticles were prepared by conjugating IR783 to gelatin, using carbodiimide chemistry, thereafter incorporating iron oxide, and finally being coated with human serum albumin.34 IR780-based nanoparticles have also been specifically developed for targeting and imaging brain tumors (Fig. 4A). IR780-based nanoparticles were prepared by loading IR780 into liposomes for the subsequent development of IR780 phospholipid micelle, which had sizes of 95 nm and 26 nm, respectively. Cellular uptake in glioma cells was evaluated and the mitochondrial uptake profile was found to be excellent for both nanoparticles and free dye. In vivo imaging showed increased accumulation of IR780 phospholipid micelles in U87MG glioma orthotopic tumors, in comparison to IR780-liposomes (Fig. 4B). This, therefore, indicated that IR780 phospholipid micelles have a malignant brain tumor-targeting effect and could be used in future clinical studies.35 In another study, IR784 was PEGylated to form GUMBOS (Group of Uniform Materials Based on Organic Salts), which were nano- or mesoscale particles 100 to 220 nm in size. GUMBOS showed a large Stokes shift of 122 nm, as measured by UV spectroscopy, which is a positive feature for in vivo imaging where long wavelength dyes offer better light penetration.36
NIRF imaging holds great promise for tumor detection as it presents numerous benefits for bioimaging; however, a disadvantage of NIRF for bioimaging is its very low spatial resolution. Combining NIRF with either magnetic resonance imaging (MRI) or PET is a good option to overcome any disadvantages since MRI and PET provide higher resolution. The NIR dye IR825 was previously used to synthesize nanoscale metal-organic particles (NMOPs), composed of Mn2+ and polydopamine (PDA), which form core-shell nanoparticles with an external polyethylene glycol (PEG) layer. Mn-IR825@PDA-PEG NMOPs offer a T1-weighted MRI through mediation by Mn2+, optical imaging, and the photothermal properties of IR825 (Fig. 5A). The nanoparticle is supposed to have excellent tumor-targeting properties with a rapid renal excretion behavior, therefore imparting multifunctional imaging use for the Mn-IR825@PDA-PEG nanoparticle.37
NIR dyes can be conjugated with gadolinium (Gd) for dual imaging purposes. The NIR dye MHI-148 has been conjugated to different types of porous Gd silicate-related nanoparticles (porous Gd silicate@mSiO2) to impart T1-T2 effects. Taken in combination with the tumor-targeting and NIR fluorescence effects of MHI-148, this finally provided a multimodal capability to the nanoparticle.38 NIR dye IR825 was synthesized, combined with human serum albumin (HSA) and covalently linked to DTPA-Gd to inhibit tumor metastasis by targeting to the sentinel lymph nodes (SLNs). The HSA-Gd-IR825 nanocomplex was found to be capable of identifying sentinel lymph nodes through lymphatic circulation. This, in turn, promoted the idea of the ‘photothermal ablation assisted surgery’ strategy with assistance from MRI and NIR imaging.39 In a separate study, a MHI-148 DOTA conjugate was incorporated to 64Cu (PC-1001) for multimodal breast cancer targeted imaging. In vivo studies indicated that the nanocomplex accumulated at very high levels in the tumor, with a 4.3-fold enhancement at 24 hours and a 5.8-fold enhancement at 48 hours.40
Additional studies using IR825, found the dye formed nanocomplexes with the cationic polymer polyallylamine hydrochloride (PAH). The resulting nanocomplexes were termed J-aggregates and were found to have red-shifted fluorescence properties suitable for in vivo NIR imaging. This system was subsequently complexed with ultra-small iron oxide nanoparticles (IONP) and then PEGylated to form IR825@PAH-IONP-PEG composite nanoparticles that had NIR imaging properties, T2 contrast, and finally photothermal abilities when irradiated with a 915 nm laser (Fig. 5B). MRI analysis following intravenous injection of these particles showed high particle accumulation in the 4T1 tumor model, which was mediated by passive targeting and finally in vivo photothermal tumor ablation.41
Photoacoustic imaging (PAI) is a method that analyzes optical energy density through inference from ultrasonic waves. Ultrasonic waves are emitted by photoacoustic imaging probes after laser absorption in the tissue.42 The PAI property of the heptamethine cyanine dye IR780 is considered useful as it is more stable than ICG, which is conventionally used as an optical/photoacoustic agent. A multifunctional nanocomplex containing poly (ɛ-caprolactone) conjugated to helical poly (phenyl isocyanide) and loaded with IR780/camptothecin, was thus synthesized with NIRF optical/PAI properties along with PTT/chemotherapeutic effects, as confirmed through in vivo studies.43
Pulmonary gene delivery with imaging capabilities were previously tested using ICG-pDNA/PEI complexes, but failed due to the loss of fluorescence efficiency in the presence of PEI and the high absorption rate of ICG in lung tissue (15 minutes evaluation period). In order to obtain excellent deposition in lung tissue and thereby evaluate gene expression, a PEG-NIRF agent containing NIR dye IR820 was complexed with pDNA/PEI (PEG-NIRF/complex). A PEG-NIRF/complex pulmonary localization study in mice established that 20% of locally delivered samples remained in lung tissue for 60 minutes, which was significantly higher than the 15 minutes for the ICG-pDNA/PEI complex. A lung deposition and gene expression correlation study found that after 60 minutes, target gene expression was significant.44 This study therefore highlights the importance of using a heptamethine dye, as opposed to an ICG dye, for improved antitumor efficacy when using a gene delivery strategy.
The chemotherapeutic actions of heptamethine dyes for killing cancer cells are based on its mitochondrial accumulation ability in cancer cells above a threshold level, which deregulates mitochondrial metabolism/permeabilization, thereby activating the endogenous apoptosis pathway that then leads to cancer cell death.454647 Drug conjugated heptamethine dyes have also been studied for tumor-targeted drug delivery. IR783 conjugated to gemcitabine (NIRG) was evaluated and was aimed at the treatment of brain tumors and brain tumor metastases. Preferential accumulation of NIRG in U87 brain tumors was observed with maximum accumulation after 24 hours. NIRG treatment significantly reduced tumor size thus indicating the potential capacity of utilizing brain tumor-targeting dye-drug conjugates for cancer chemotherapy.48
Another potential use of heptamethine dyes is to harness their photothermal abilities owing to their absorbance in the NIR range with concomitant emission of heat. IR780 iodide, a near-infrared (near-IR) fluorescent dye, is a lipophilic dye that can be loaded in amphiphilic conjugates. This helps protect the dye from non-specific toxicity and enhances its photothermal efficiency (Fig. 6A).
IR780 was previously loaded into the phospholipid mimicking amphiphilic homopolymer poly (12-(methacryloyloxy) dodecyl phosphorylcholine) (PMDPC), which was synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization. The size of the resultant nanoparticle was 159.9 nm. In vivo accumulation of PMDPC-IR780 in tumors was found to be greater than that of free IR780 in pancreatic tumor (BxPC-3) bearing mice. In addition, the in vitro photothermal effect of the nanoparticle was shown in BxPC-3 cells when irradiated with a 0.8 W/cm2 laser.49 In a separate study, PTT was used to treat bladder cancer. Hyaluronic acid was conjugated to amine modified IR780 forming amphiphilic conjugates that were then loaded with IR780 to obtain an enhanced photothermal effect along with CD44+-mediated tumor-targeted therapy. Additionally, the nanoparticle also exhibited a fluorescence on/off property in the presence of high hyaluronidase enzyme activity. The tumor reduction effect using PTT was studied and found to be highly effective at 20 mg/kg of HA-IR780 nanoparticles (Fig. 6B), with complete removal of the tumor in an orthotopic bladder cancer model.50
IR825 loaded in PEG-grafted poly (maleic anhydridealt-1-octadecene) (C18PMH-PEG) was synthesized to form micelle nanoparticles (IR825-PEG) with a median size of 25 nm. Fluorescence was almost completely quenched inside the micelle structure due to the intermolecular interaction, while retaining ultra-high photothermal efficiency without any toxicity to the treated animals.51 In another study, hydrophobic IR780 was modified to an even more hydrophobic structure by adding 13 carbon chains to one side and PEG2000 conjugation on the other to form a novel self-assembled particle capable of forming a soluble micelle (PEG-IR780-C13) with an average size of 116 nm. The tumor-targeting ability of these micelle nanoparticles was tested in CT-26 xenograft tumors and was shown to have high accumulation in tumors with photothermal ablation of tumor cells when irradiated with a laser set to 808 nm.52
Photosensitizers are responsible for reactive oxygen species (ROS) production and free radicals in the region of the tumor (Fig. 7), which are toxic for tumor cells and lead to subsequent tumor reduction.5455 Heptamethine dyes, such as IR125, IR780, IR813, and IR820, were studied for their photodynamic effect in HeLa cells, with IR125 and IR813 found to be particularly phototoxic. IR125 showed dark toxicity of 75% and cell toxicity of 95% after irradiation at a concentration of 500 nM, while IR813 showed dark toxicity of 82 % and cell toxicity of 93% after irradiation at the same concentration. The reason for the phototoxcity is the interaction of the sensitizer with molecular oxygen through a triplet-triplet annihilation process that in turn generates ROS.26
IR808, which is an analogue of IR780, was synthesized to show the photodynamic efficiency of IR808;56 however, the same group of researchers modified IR808 to show improved photodynamic efficiency. They developed an n-butyl ester derivative of IR808, namely IR808DB, and tested its anticancer effect in A549 cells (human lung cancer). IR808DB showed a 30-times greater anticancer effect than IR808. The photodynamic effect of IR808DB was tested in rTDMC cells and found to have high cell toxicity compared to dark toxicity when irradiated.57 In another study, NIR dye IR780 was used for cancer imaging and phototherapy by loading the dye inside amphiphilic micelles, which were based on D-α-tocopheryl polyethylene glycol succinate (TPGS) and D-α-tocopheryl succinate (TOS). The size of the resulting nanoparticles was found to be 115 nm, which was appropriate for passive targeted accumulation in tumor tissues. The DCF-DA assay was used to study ROS release in MCF-7 cells after irradiation with a laser. The fluorescence of micelle loaded with IR780 was significantly higher than the control, indicating the ROS release was higher.58
Sonodynamic therapy (SDT) is a new method in which tissue penetrating low-intensity ultrasound (US) and sonosensitizers are combined to produce ROS in the tumor region, which offers a non-invasive approach to eradicating the tumor.59 IR780 iodide was recently used to function as the sonosensitiser for sonodynamic therapy (Fig. 8). This study showed that treatment with different concentrations of IR780 led to the release of high amounts of ROS in the 4T1 breast cancer cell line, along with a decrease in cell viability. An in vivo anti-tumor study also showed a significant reduction of the 4T1 breast tumor. Usage of heptamethine dyes as sonodynamic and photothermal agents for therapy is thus a very viable strategy for cancer therapy.60
A Combination of photothermal therapy and chemotherapy is a promising strategy for treating cancer. A nanoparticle system loaded with chemotherapeutic cargo and a photothermal agent can be targeted to the tumor region and synergistically suppress tumors with much greater efficiency than a single drug strategy. ICG is the current photothermal agent of choice for PTT/chemotherapy since it is FDA approved and shows good photothermal efficiency.61 Recently, however, heptamethine dyes have been used instead of ICG because of their higher propensity to accumulate in tumors.
The dual-functional thermosensitive bubble-generating liposome (BTSL) was designed with an ability to target folic acid (FA)-overexpressing cancer cells. It was loaded with IR780 in its hydrophobic compartment and doxorubicin/ammonium bicarbonate (NH4HCO3) in its hydrophilic compartment. The triggered release of DOX was achieved by photothermal-mediated decomposition of the lipid bilayer by converting ammonium bicarbonate to CO2 bubbles, which causes a burst release phenomenon in cancer cells. An in vivo antitumor study showed the efficient reduction of tumors overexpressing FA through the combined action of PTT and chemotherapy.62 In a separate study, a smart, temperature-sensitive-liposome with DOX and IR780 (DITSL) was synthesized with a trigger release property through photothermal action (Fig. 9). DITSL nanoparticles showed release of the chemotherapeutic drug at temperatures higher than 42℃ but not below 37℃ (normal body temperature), which is explained by the phase transition temperature of the lipid bilayer. For tumor treatment, 4T1 breast cancer tumors were selected and an intra-tumoral injection was administered at 20 µg/20 µg of DOX/IR780 and 1 W/cm2 808 nm laser application in the tumor region. A significant reduction of tumor was observed by comparison to control groups.63
In an additional study, p (NIPAAM-co-PEGMEA)-b-PCL was loaded with IR780 and the hydrophobic heat shock protein 90 inhibitor (17-allylamino-17-demethoxygeldanamycin) was synthesized with an average particle diameter of 200 nm. The in vitro and in vivo effect of these nanoparticles was studied in HCT-116 cells and in HCT-116 tumors induced in SCID mice by applying the laser set to 0.8 W/cm2 for 2 minutes to the tumor region after migration of the micelles. Compared to unloaded micelles, co-loaded micelles showed effective tumor reduction.64
Another type of combination therapy is PTT/PDT, where the therapeutic agent and photosensitizer are the NIRF heptamethine dyes. In this case, a multifunctional nanocarrier encapsulates both chlorin e6 (Ce6) and acts as a photosensitizer. The so-called theranosome (TNS) encapsulates Ce6 by protecting it from photodegradation. The TNS, after reaching the tumor site, is degraded by the photothermal ablation effect of IR780 thereby releasing Ce6 and simultaneously recovering phototoxic activity. The mitochondrial targeting effect of TNS is enhanced by adding triphenylphosphonium (TPP), which is a mitochondrial targeting agent, to the nanoparticle system. In vitro tests revealed that the TNS/TPP system has a very high toxic effect to tumor cells compared to normal cells due to combined PTT/PDT effect.65
The dual properties of IR780 to act as a PTT/PDT agent was reported with regard to human serum albumin-loaded IR780 (HSA-IR780) nanoparticles. The main feature of these nanoparticles was to enhance IR780 solubility (almost 1,000 times) and decrease toxicity in mice. The in vivo tumor reduction study was conducted with an intravenous injection of HSA-IR780 nanoparticles followed by laser irradiation (1 W/cm2) 24 hours later in CT-26 tumor-induced BALB/c mice. Tumor reduction was only observed for the nanoparticle injected and laser irradiated model. This study showed the dual properties of IR780 to act as both a PTT and PDT agent.66
A NIR photosensitizer for targeting mitochondria for simultaneous cancer PTT and PDT was prepared. Heptamethine cyanine dye was modified in this work to form various analogs with N-alkyl side chains in the heptamethine dye core which has PS activity. This new design of heptamethine dye has PTT and PDT effects which are targeted to mitochondria. It can act as a good phototherapy (PTT and PDT) agent as mitochondria are vulnerable to both PTT and PDT. This was tested in vitro and in vivo and found to be effective in both.67
In another work, NIR heptamethine dye IR-780 was used for PTT/PDT by modification with transferrin protein to render the dye hydrophilic and multifunctional (Fig. 10A). The main key point in this work is the application of a single laser wavelength for both PTT and PDT. The size of the nanoparticles was 65 nm on average. In vitro phototherapy studies showed effective tumor accumulation and cell death in the CT-26 cell line (Fig. 10B). The in vivo tumor reduction study in CT-26 tumor induced mice also showed effective therapeutic effects, possibly due to the PTT/PDT combine effect as stated earlier (Fig. 10C).68
Heptamethine cyanine dyes have gained interest due to their tumor specific accumulation and theranosis potential. Various analogues of heptamethine dyes have been synthesized and all have excellent bioavailability, biostability, and a fluorescent property as common features. Some of the dyes have been tweaked with a few molecular modifications to enhance cancer toxicity, hydrophobicity, or cancer specificity. Apart from functional modifications, structural changes in dyes have also been tried out for increased stability and photosensitivity. Heptamethine dyes have been used as a part of a nanocomplex system that offers multimodal imaging, photothermal, photodynamic, and combinatorial therapeutic approaches. In the future, heptamethine cyanine dyes can be considered as suitable choices for imaging and therapeutic applications.
Figures and Tables
FIG. 2
Different NIRF heptamethine dye structures. Figure reproduced with permission from (25) and (26). Copyright © 2017 Impact Journals and MDPI AG.
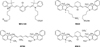
FIG. 3
Preferential uptake and retention of MHI-148. (A) MHI-148 chemical structure. (B) Ex vivo NIR fluorescence imaging showed increased MHI-148 dye uptake by different types of canine spontaneous tumors (blue arrows) as compared to adjacent normal tissues (white arrows). (C) Preferential uptake of NIRF dye in gastric cancer tissues relative to that in gastric tissues. In vivo NIRF imaging of mice bearing either orthotopic luc-tagged gastric tumor xenografts (left) or gastric ulcers (right). (D) NIRF imaging of clinical gastric cancer tissues. Schematic outlining the experimental procedures for NIRF imaging of freshly resected clinical gastric tumor tissues (left). Gross morphology and NIRF imaging of representative tumor tissues surgically resected from one of three gastric cancer patients. Representative images are presented in all panels. Original magnification: 4×; scale bars represent 4 mm. Figure images and the accompanying legend are reproduced with permission from (2530). Copyright © 2017 Impact Journals.
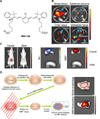
FIG. 4
IR780-liposome and IR780-phospholipid micelle developed for NIRF optical imaging. (A) Structure of IR780 iodide free dye, an IR780-liposome, and IR780-phospholipid micelle. (B) Real-time NIRF imaging of IR780-phospholipid micelles using the glioma spontaneous mouse model. Bioluminescence imaging (BLI) indicates the location and status of the U87MG ectopic tumor. Figure images and accompanying legend are reproduced with permission from (35). Copyright © 2017 American Chemical Society.

FIG. 5
Synthesis, in vivo MRI, and photothermal therapy of (A) Mn-IR825@PDA-PEG and (B) IR825@PAH-IONP-PEG. Figure images and accompanying legend are reproduced with permission from (3741). Copyright © 2012 American Chemical Society and John Wiley & Sons, Inc.
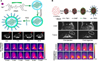
FIG. 6
Photothermal therapy of cancer cells (A) Laser-induced thermal damage to cancer cells after accumulation of NIRF probes. (B) Synthesis of IR780 loaded PMDPC-IR780 micelle nanoparticles and its application in photothermal therapy. Figure images and accompanying legend are reproduced with permission from (4953). Copyright © 2017 Dove Press Ltd and American Chemical Society.

FIG. 7
Photodynamic therapy by light-induced ROS release and damage to cancer cells after NIRF probe accumulation. Figure images and accompanying legend are reproduced with permission from (53). Copyright © 2017 Dove Press Ltd.

FIG. 8
Sonodynamic therapy using IR780 (A) Schema showing the action of IR780 in releasing ROS using a sonodynamic transducer. Upon receiving US, IR780 that had accumulated in the tumor cells would receive US energy. When in the excited-state, IR780 restored back to the ground-state and releasing energy; 1O2 and H2O absorb the released energy and changed into 1O2 and H2O2. The superfluous 1O2 and H2O2 would subsequently cause the apoptosis and necrosis of tumor cells. (B) Quantification of ROS release by the DCF-DA assay in 4T1 cancer cells for 1O2 (a), H2O2 (b), and ·OH (c). (C) Cell viability analysis of 4T1 breast cancer cells incubated with PBS, 4µM, 10µM, or 16µM of IR780. 4T1 breast cancer cells were incubated with PBS or IR780 and then administered with US for 0 s, 20 s, or 40 s. Twenty-four hours later, the levels of 1O2 were evaluated. (D) Photograph of 4T1 tumors removed from mice 30 days after the tumor-bearing mice were treated by SDT with IR780. Figure images and accompanying legend are reproduced from (60). Copyright © 2017 Macmillan Publishers Limited.
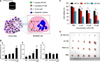
FIG. 9
DITSL nanoparticles for PTT/Chemotherapy. (A) Schematic diagram of DOX release from DITSL under NIR-laser irradiation. The liposome membrane temperature would increase when the NIR-laser irradiation was applied. Destruction of the liposome membrane occurs when the membrane temperature reaches 42℃. (B) Schematic diagram of DITSL preparation. (C) The representative infrared photothermal images of tumors following laser irradiation. Figure images and accompanying legend are reproduced from (63). Copyright © 2017 Ivyspring International Publisher.

FIG. 10
Schematic of Tf-IR780 nanoparticle preparation, in vitro phototherapy, and biodistribution mediated by Tf-IR780 NPs. (A) Transferrin self-assembly with IR-780 with the help of dithiothreitol (DTT) to form Tf-IR780 NPs (B) Fluorescence images of CT-26 cells expressing singlet oxygen indicated by H2DCFDA staining for detection under photoirradiation (1 W/cm2; 808 nm) for 5 min (Scale bar=20µm). (C) In vivo fluorescence imaging in mice bearing CT-26 tumors administered with Tf-IR780 NPs (0.3 mg/kg, IR780). Figure images and accompanying legend are reproduced from (68). Copyright © 2017 Macmillan Publishers Limited.
ACKNOWLEDGEMENTS
This research was supported by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Future Planning (2015R1A2A2A01007798)
References
1. Jian WH, Yu TW, Chen CJ, Huang WC, Chiu HC, Chiang WH. Indocyanine green-encapsulated hybrid polymeric nanomicelles for photothermal cancer therapy. Langmuir. 2015; 31:6202–6210.

2. Ma Y, Tong S, Bao G, Gao C, Dai Z. Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy. Biomaterials. 2013; 34:7706–7714.

3. Miki K, Inoue T, Kobayashi Y, Nakano K, Matsuoka H, Yamauchi F, et al. Near-infrared dye-conjugated amphiphilic hyaluronic acid derivatives as a dual contrast agent for in vivo optical and photoacoustic tumor imaging. Biomacromolecules. 2015; 16:219–227.

4. Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X. A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. Nat Commun. 2015; 6:10081.

5. Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J Pharm Sci. 2013; 102:6–28.

6. Zhao P, Zheng M, Luo Z, Gong P, Gao G, Sheng Z, et al. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Sci Rep. 2015; 5:14258.

7. Zhao P, Zheng M, Yue C, Luo Z, Gong P, Gao G, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014; 35:6037–6046.

8. Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011; 8:447–456.

9. Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016; 10:2436–2446.

10. GhavamiNejad A, SamariKhalaj M, Aguilar LE, Park CH, Kim CS. pH/NIR light-controlled multidrug release via a mussel-inspired nanocomposite hydrogel for chemo-photothermal cancer therapy. Sci Rep. 2016; 6:33594.

11. Jung HS, Han J, Lee JH, Lee JH, Choi JM, Kweon HS, et al. Enhanced NIR radiation-triggered hyperthermia by mitochondrial targeting. J Am Chem Soc. 2015; 137:3017–3023.

12. Kim J, Oh J, Kang HW, Feldman MD, Milner TE. Photothermal response of superparamagnetic iron oxide nanoparticles. Lasers Surg Med. 2008; 40:415–421.

13. Wang T, Wang D, Yu H, Wang M, Liu J, Feng B, et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano. 2016; 10:3496–3508.

14. Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q, et al. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials. 2016; 81:114–124.

15. Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, et al. Biomineralization-Inspired Synthesis of Copper Sulfide-Ferritin Nanocages as Cancer Theranostics. ACS Nano. 2016; 10:3453–3460.

16. Wu M, Zhang D, Zeng Y, Wu L, Liu X, Liu J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology. 2015; 26:115102.

17. Zhang N, Xu X, Zhang X, Qu D, Xue L, Mo R, et al. Nanocomposite hydrogel incorporating gold nanorods and paclitaxel-loaded chitosan micelles for combination photothermal-chemotherapy. Int J Pharm. 2016; 497:210–221.

18. Zhou F, Wu S, Wu B, Chen WR, Xing D. Mitochondria-targeting single-walled carbon nanotubes for cancer photothermal therapy. Small. 2011; 7:2727–2735.

19. Zhou Z, Sun Y, Shen J, Wei J, Yu C, Kong B, et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials. 2014; 35:7470–7478.

20. Shi C, Wu JB, Pan D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J Biomed Opt. 2016; 21:50901.

21. Yang X, Shao C, Wang R, Chu CY, Hu P, Master V, et al. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J Urol. 2013; 189:702–710.

22. Yang X, Shi C, Tong R, Qian W, Zhau HE, Wang R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010; 16:2833–2844.

23. Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011; 32:7127–7138.

24. Yuan J, Yi X, Yan F, Wang F, Qin W, Wu G, et al. Near infrared fluorescence imaging of prostate cancer using heptamethine carbocyanine dyes. Mol Med Rep. 2015; 11:821–828.

25. Shi C, Wu JB, Chu GC, Li Q, Wang R, Zhang C, et al. Heptamethine carbocyanine dye-mediated near-infrared imaging of canine and human cancers through the HIF-1α/OATPs signaling axis. Oncotarget. 2014; 5:10114–10126.

26. Conceicao DS, Ferreira DP, Ferreira LF. Photochemistry and cytotoxicity evaluation of heptamethinecyanine Near Infrared (NIR) dyes. Int J Mol Sci. 2013; 14:18557–18571.

27. Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X, et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials. 2010; 31:6612–6617.

28. Wang Y, Liu T, Zhang E, Luo S, Tan X, Shi C. Preferential accumulation of the near infrared heptamethine dye IR-780 in the mitochondria of drug-resistant lung cancer cells. Biomaterials. 2014; 35:4116–4124.

29. Wu JB, Shao C, Li X, Shi C, Li Q, Hu P, et al. Near-infrared fluorescence imaging of cancer mediated by tumor hypoxia and HIF1α/OATPs signaling axis. Biomaterials. 2014; 35:8175–8185.

30. Zhao N, Zhang C, Zhao Y, Bai B, An J, Zhang H, et al. Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget. 2016; 7:57277–57289.

31. Zhang E, Luo S, Tan X, Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials. 2014; 35:771–778.

32. Buxhofer-Ausch V, Secky L, Wlcek K, Svoboda M, Kounnis V, Briasoulis E, et al. Tumor-specific expression of organic anion-transporting polypeptides: transporters as novel targets for cancer therapy. J Drug Deliv. 2013; 2013:863539.
33. Singh AK, Hahn MA, Gutwein LG, Rule MC, Knapik JA, Moudgil BM, et al. Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. Int J Nanomedicine. 2012; 7:2739–2750.
34. Corem-Salkmon E, Perlstein B, Margel S. Design of near-infrared fluorescent bioactive conjugated functional iron oxide nanoparticles for optical detection of colon cancer. Int J Nanomedicine. 2012; 7:5517–5527.
35. Li S, Johnson J, Peck A, Xie Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J Transl Med. 2017; 15:18.

36. Lu C, Das S, Magut PK, Li M, El-Zahab B, Warner IM. Irradiation induced fluorescence enhancement in PEGylated cyanine-based NIR nano- and mesoscale GUMBOS. Langmuir. 2012; 28:14415–14423.

37. Yang Y, Liu J, Liang C, Feng L, Fu T, Dong Z, et al. Nanoscale metal-organic particles with rapid clearance for magnetic resonance imaging-guided photothermal therapy. ACS Nano. 2016; 10:2774–2781.

38. Yeh CS, Su CH, Ho WY, Huang CC, Chang JC, Chien YH, et al. Tumor targeting and MR imaging with lipophilic cyanine-mediated near-infrared responsive porous Gd silicate nanoparticles. Biomaterials. 2013; 34:5677–5688.

39. Chen Q, Liang C, Wang X, He J, Li Y, Liu Z. An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 2014; 35:9355–9362.

40. Xiao L, Zhang Y, Yue W, Xie X, Wang JP, Chordia MD, et al. Heptamethine cyanine based (64)Cu-PET probe PC-1001 for cancer imaging: synthesis and in vivo evaluation. Nucl Med Biol. 2013; 40:351–360.

41. Song X, Gong H, Liu T, Cheng L, Wang C, Sun X, et al. J-aggregates of organic dye molecules complexed with iron oxide nanoparticles for imaging-guided photothermal therapy under 915-nm light. Small. 2014; 10:4362–4370.

42. Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Methods. 2016; 13:639–650.

43. Shi S, Liu Y, Chen Y, Zhang Z, Ding Y, Wu Z, et al. Versatile pH-response micelles with high cell-penetrating helical diblock copolymers for photoacoustic imaging guided synergistic chemo-photothermal therapy. Theranostics. 2016; 6:2170–2182.

44. Okuda T, Kobayashi Y, Yanamoto S, Okamoto H. PEG conjugation of a near-infrared fluorescent probe for noninvasive dual imaging of lung deposition and gene expression by pulmonary gene delivery. J Drug Target. 2012; 20:801–812.

45. Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000; 92:1042–1053.

46. Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010; 9:447–464.

47. Zhang E, Zhang C, Su Y, Cheng T, Shi C. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug Discov Today. 2011; 16:140–146.

48. Wu JB, Shi C, Chu GC, Xu Q, Zhang Y, Li Q, et al. Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials. 2015; 67:1–10.

49. Chen Y, Li Z, Wang H, Wang Y, Han H, Jin Q, et al. IR-780 loaded phospholipid mimicking homopolymeric micelles for near-IR imaging and photothermal therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2016; 8:6852–6858.

50. Lin T, Yuan A, Zhao X, Lian H, Zhuang J, Chen W, et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017; DOI: 10.1016/j.actbio.2017.02.021. [Epub ahead of print].

51. Cheng L, He W, Gong H, Wang C, Chen Q, Cheng Z, et al. PEGylated micelle nanoparticles encapsulating a non-fluorescent near-infrared organic dye as a safe and highly-effective photothermal agent for in vivo cancer therapy. Adv Funct Mater. 2013; 23:5893–5902.

52. Yuan A, Qiu X, Tang X, Liu W, Wu J, Hu Y. Self-assembled PEG-IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials. 2015; 51:184–193.

53. Yi X, Wang F, Qin W, Yang X, Yuan J. Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field. Int J Nanomedicine. 2014; 9:1347–1365.

54. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61:250–281.

56. Tan X, Luo S, Wang D, Su Y, Cheng T, Shi C. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials. 2012; 33:2230–2239.

57. Luo S, Tan X, Qi Q, Guo Q, Ran X, Zhang L, et al. A multifunctional heptamethine near-infrared dye for cancer theranosis. Biomaterials. 2013; 34:2244–2251.

58. Pais-Silva C, de Melo-Diogo D, Correia IJ. IR780-loaded TPGSTOS micelles for breast cancer photodynamic therapy. Eur J Pharm Biopharm. 2017; 113:108–117.

59. Wan GY, Liu Y, Chen BW, Liu YY, Wang YS, Zhang N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016; 13:325–338.

60. Li Y, Zhou Q, Deng Z, Pan M, Liu X, Wu J, et al. IR-780 dye as a sonosensitizer for sonodynamic therapy of breast tumor. Sci Rep. 2016; 6:25968.

61. Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, et al. Single-step assembly of DOX/ICG loaded lipid--polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013; 7:2056–2067.

62. Guo F, Yu M, Wang J, Tan F, Li N. Smart IR780 theranostic nanocarrier for tumor-specific therapy: hyperthermia-mediated bubble-generating and folate-targeted liposomes. ACS Appl Mater Interfaces. 2015; 7:20556–20567.

63. Yan F, Duan W, Li Y, Wu H, Zhou Y, Pan M, et al. NIR-laser-controlled drug release from DOX/IR-780-loaded temperature-sensitive-liposomes for chemo-photothermal synergistic tumor therapy. Theranostics. 2016; 6:2337–2351.

64. Peng CL, Chen YI, Liu HJ, Lee PC, Luo TY, Shieh MJ. A novel temperature-responsive micelle for enhancing combination therapy. Int J Nanomedicine. 2016; 11:3357–3369.
65. Guo F, Yu M, Wang J, Tan F, Li N. The mitochondria-targeted and IR780-regulated theranosomes for imaging and enhanced photodynamic/photothermal therapy. RSC Adv. 2016; 6:11070–11076.

66. Jiang C, Cheng H, Yuan A, Tang X, Wu J, Hu Y. Hydrophobic IR780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomater. 2015; 14:61–69.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download