Four major melanoma subtypes are commonly recognized: lentigo maligna melanoma (LMM), superficial spreading melanoma (SSM), acral lentiginous melanoma (ALM) and nodular melanoma (NM).1 In his unifying concept, Ackerman argues that no clinical, histological and biological criteria can been established for LMM, SSM, ALM or NM.2 The Author concludes that the best histological diagnosis should be simply “melanoma”, without referencing the artificially designated histotype, while noting the anatomic site.2 Primary melanoma evolves through logical tumor progression phases (Fig. 1).3 At first, transformed melanocytes proliferate above the epidermal basement membrane with a lentiginous or pagetoid pattern, the so-called intra-epidermal radial growth phase (RGP), corresponding to in situ LMM, SSM or ALM.4 The subsequent step is dermal invasion in the absence of tumorigenic nodules or papules and regression (non-tumorigenic micro-invasive RGP without regression).5 This phase may be followed by a tumorigenic vertical growth phase (VGP) with deeper extension into the dermis or subcutis and metastatic capacity (Fig. 2).6 An exception is represented by NM, which either skips the RGP or where the RGP is rapidly overrun by VGP.7 Besides clear-cut Ms, there are also melanocytic neoplasms with uncertain biological behaviors, which include new clinico-pathological entities more and more adopted in dermatopathology like Superficial Atypical Melanocytic Proliferation of Uncertain Significance (SAMPUS), MELanocytic Tumor of Uncertain Malignant Potential (MELTUMP), micro-invasive RGP (≤1 mm) with regression (0.75-1.00 mm in depth; >75% in volume) of uncertain tumorigenic potential at diagnosis.891011 A final diagnosis of SAMPUS or MELTUMP should always be preceded by an accurate description of the lesion with an attempt at morphological labeling, such as atypical Spitz nevus, atypical cellular blue nevus, deep penetrating nevus, pigmented epithelioid melanocytoma, dysplastic nevus with atypia or any nevus with atypical features.8 On the basis of the tumor progression, we therefore propose a new classification for the non-benignant melanocytic lesions (Table 1). It fits well with the American Joint Committee on Cancer (AJCC) staging system,12 and it should be used in all epidemiological, clinical and histological studies on M. This classification allows for explanation of the prognostic variability recorded in thin melanoma, and to identify the patients as candidates for sentinel node biopsy. In fact, in our experience, the thin melanomas with the worst prognosis are the early (≤1 mm) invasive VGP and the micro-invasive RGP with regression,6 where the regression area most probably incorporated a VGP clone.13 The sentinel node biopsy should be reserved to these two above-mentioned cases of thin melanoma, thick melanoma, and MELTUMP.
Figures and Tables
FIG. 1
Schematic representation of the progression from normal skin to invasive malignant melanoma. (A) Normal skin with melanocytes (smaller grey bullets) regularly distributed at the basis of the epidermis (black line). (B) Epidermis with basal hyperplastic melanocytes. (C) Epidermis with basal atypical melanocytes (larger grey bullets). (D) Intra-epidermal radial growth phase of primary malignant melanoma with lentiginous pattern. (E) Intra-epidermal radial growth phase of primary malignant melanoma with pagetoid pattern. (F) Micro-invasive radial growth phase of primary malignant melanoma with lentiginous pattern (single melanoma cells are present in the papillary dermis). (G) Micro-invasive radial growth phase of primary malignant melanoma with pagetoid pattern (small clusters of melanoma cells are present in the papillary dermis). (H) Invasive (early if ≤1 mm in thick) vertical growth phase of primary cutaneous malignant melanoma (large clusters of melanoma cells are present in the papillary dermis and beyond).
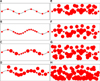
FIG. 2
Histopathological exemplification of the radial growth phase (A: hematoxylin/eosin, 4×) and of the vertical one (B: hematoxylin/eosin, 4×) in primary cutaneous malignant melanoma.
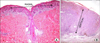
TABLE 1
The borderline and malignant melanocytic lesions of the skin can be subdivided, according to the Breslow thickness, in SAMPUS and thin melanoma (≤1 mm) or MELTUMP and thick melanoma (>1 mm). The SAMPUS and MELTUMP categories are characterized by unknown biological behaviour, which reflects diagnostic uncertainty not excluding malignancy. The intra-epidermal radial growth phase and the micro-invasive radial growth phase without regression of thin melanoma are devoid of tumorigenic potential, while the micro-invasive radial growth phase with regression is burdened by an uncertain metastatic potential. The early invasive vertical growth phase of thin melanoma and the subcategories of invasive vertical growth phase of thick melanoma show all tumorigenic potential, directly correlated to the depth of invasion (pTis, pT1, pT2, pT3, pT4 and a/b specifications are adapted from the AJCC staging system)

References
3. Roncati L, Piscioli F, Pusiol T. Sentinel lymph node in thin and thick melanoma. Klin Onkol. 2016; 29:393–394.
4. Piscioli F, Pusiol T, Roncati L. Wisely choosing thin melanomas for sentinel lymph node biopsy. J Am Acad Dermatol. 2017; 76:e25.

5. Madu MF, Wouters MW, van Akkooi AC. Sentinel node biopsy in melanoma: current controversies addressed. Eur J Surg Oncol. 2016; DOI: 10.1016/j.ejso.2016.08.007. [Epub ahead of print].

6. Piscioli F, Pusiol T, Roncati L. Histopathological determination of thin melanomas at risk for metastasis. Melanoma Res. 2016; 26:635.

7. Piscioli F, Pusiol T, Roncati L. Nowadays a histological sub-typing of thin melanoma is demanded for a proper patient management. J Plast Reconstr Aesthet Surg. 2016; 69:1563–1564.

8. Pusiol T, Piscioli F, Speziali L, Zorzi MG, Morichetti D, Roncati L. Clinical features, dermoscopic patterns, and histological diagnostic model for melanocytic tumors of uncertain malignant potential (MELTUMP). Acta Dermatovenerol Croat. 2015; 23:185–194.
9. Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology. 2004; 36:428–434.

10. Piscioli F, Pusiol T, Roncati L. Diagnostic approach to melanocytic lesion of unknown malignant potential. Melanoma Res. 2016; 26:91–92.

11. Piscioli F, Pusiol T, Roncati L. Diagnostic disputes regarding atypical melanocytic lesions can be solved by using the term MELTUMP. Turk Patoloji Derg. 2016; 32:63–64.

12. Piscioli F, Pusiol T, Roncati L. Thin melanoma subtyping fits well with the American Joint Committee on Cancer staging system. Melanoma Res. 2016; 26:636.

13. Roncati L, Piscioli F, Pusiol T. Surgical outcomes reflect the histological types of cutaneous malignant melanoma. J Eur Acad Dermatol Venereol. 2016; DOI: 10.1111/jdv.14023. [Epub ahead of print].





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download