Abstract
The present study investigated the prognostic factors predicting survival of patients with sepsis and acute kidney injury (AKI) undergoing continuous renal replacement therapy (CRRT). This retrospective observational study included 165 sepsis patients treated with CRRT. The patients were divided into two groups; the survivor group (n=73, 44.2%) vs. the nonsurvivor group (n=92, 55.8%). AKI was defined by the 2012 Kidney Disease: Improving Global Outcomes Clinical Practice Guidelines. We analyzed medical histories, clinical characteristics and laboratory findings of the enrolled patients when they started CRRT. In addition, we performed binary logistic regression and cox regression analysis. In the survivor group, urine output during the first day was significantly higher compared with the nonsurvivor group (55.7±66.3 vs. 26.6±46.4, p=0.001). Patients with urine output <30 mL/hour during the 1st day showed worse outcomes than ≥30 mL/hour in the logistic regression (hazard ratio 2.464, 95% confidence interval 1.152-5.271, p=0.020) and the cox regression analysis (hazard ratio 1.935, 95% confidence interval 1.147-3.263, p=0.013). In conclusion, urine output may predict survival of septic AKI patients undergoing CRRT. In these patients, urine output <30 mL/hour during the first day was the strongest risk factor for in-hospital mortality.
Sepsis is a systemic inflammatory reaction, and may proceed to multiple organ failure including acute kidney injury (AKI).123 In critically ill patients, sepsis is closely associated with in-hospital mortality.4567 Hospital mortality was significantly increased when AKI developed in intensive care unit (ICU) patients.891011 Furthermore, septic AKI patients had a poorer prognosis than non-septic AKI patients.12
For early diagnosis and treatment of AKI, Acute Kidney Injury Network (AKIN) and Risk, Injury, Failure; Loss, End-Stage Renal Disease (RIFLE) criteria have been validated for diagnosis of AKI.13 Recently, AKI has been defined by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines.14 These definitions based on serum creatinine and urine output as surrogates for changes of glomerular filtration rate (GFR). Increased stage AKI is associated with in-hospital mortality.151617 Previous studies have also demonstrated that the mortality of critically ill patients was associated with the increased level of serum creatinine or oliguria.18 owever, clinical biomarkers predicting survival of septic AKI undergoing continuous renal replacement therapy (CRRT) have not yet been established although several biomarkers have been investigated.192021
The present study investigated the prognostic factors predicting survival of septic AKI patients undergoing CRRT.
This retrospective observational study included 165 patients with AKI and sepsis treated with CRRT between December 2012 and August 2015. The patients were divided into two groups; the survivor group (n=73, 44.2%) vs. the nonsurvivor group (n=92, 55.8%). We defined sepsis as systemic inflammatory response syndrome (SIRS) in response to an infectious process.22 AKI was defined by the 2012 KDIGO Clinical Practice Guideline.14 We analyzed medical histories, clinical characteristics and laboratory findings of the enrolled patients when they started CRRT. Categorical variables were analyzed using the Chi-square test. Parametric variables were expressed as the mean±standard deviation, and analyzed by the unpaired t-test. Binary logistic regression and cox regression analysis after adjusting for age, sex, and medical histories were also performed. Differences with values of p<0.05 were considered statistically significant.
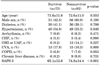
The clinical characteristics of the enrolled patients were summarized in Table 1. Of 165 patients, 92 patients (55.8%) died during hospitalization. Sex, age and underlying diseases did not differ between the survivor and nonsurvivor groups, but the simplified acute physiologic score 3 (SAPS 3) was significantly higher in the nonsurvivor group. The most common cause of sepsis was pneumonia in all of the patients in both the survivor and nonsurvivor groups (Table 2).
The laboratory findings at starting CRRT and urine output during the first day were shown in Table 3. In the survivor group, urine output during the first day was significantly higher compared with the nonsurvivor group (55.7±66.3 vs. 26.6±46.4, p=0.001). However, serum creatinine levels did not differ between the groups. White blood cell counts and platelet counts were lower but the total bilirubin level was higher in the nonsurvivor group compared with the survivor group. The value of the pH was lower in the nonsurvivor group although the bicarbonate level and anion gap did not differ between the two groups.
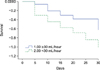
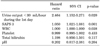
Table 4 demonstrated the results of the logistic regression analysis of risk factors for mortality in septic AKI patients with CRRT. The urine output <30 mL/hour during the 1st day was the strongest risk factor for these patients (hazard ratio 2.464, 95% confidence interval 1.152-5.271, p=0.020). The cox regression analysis also showed that the urine output <30 mL/hour during the 1st day was the risk factor for death in the septic AKI patients treated with CRRT (hazard ratio 1.935, 95% confidence interval 1.147-3.263, p=0.013) (Table 5). In the survival analysis, patients with urine outputs of ≥30 mL/hour during the 1st day showed better outcomes than those at <30 mL/hour (Fig. 1).
There are several studies working to predict the survival of the patients treated with CRRT. One of studies revealed that nonoliguria, shorter durations of CRRT, and higher baseline estimated GFR were independently associated with favorable outcomes.23 Another study demonstrated that urine output was the most important predictor of weaning from CRRT although serum creatinine was also a significant factor.24 However, the independent risk factors for early kidney recovery were urine output, duration between ICU admission to CRRT initiation and sepsis-related organ failure assessment score, but serum creatinine level did not affect the renal outcome in AKI requiring CRRT although the definitions of AKI such as AKIN, RIFLE criteria and the 2012 KDIGO Clinical Practice Guidelines primarily based on serum creatinine as a biomarker for GFR.25 Similar results were also demonstrated in the studies of AKI patients who underwent acute RRT.2627
The present study included 165 patients with AKI and sepsis who underwent CRRT, and 55.8% of the enrolled patients died regardless of age, sex and underlying diseases. In our study, urine output was significantly higher in the survivor group and decreased urine output was a strong risk factor for death of the septic AKI patients treated with CRRT. Furthermore, the patients with urine output ≥30 mL/hour showed better outcomes. However, serum creatinine levels did not differ between the two groups, and did not predict the survival of septic AKI patient requiring CRRT. Serum creatinine levels may be affected by various clinical factors such as age, gender, muscle mass, volume status, nutritional status and catabolic rate. It was reported that a higher level of serum creatinine on initiation of CRRT was paradoxically associated with better outcomes in critically ill patients with AKI.28 Furthermore, urine output may reflect changes of GFR earlier than serum creatinine.29 Studies validating urine output as a biomarker of AKI have been also reported.30 Our results suggest that the use of serum creatinine only to categorize AKI severity may miss the burden of AKI.
In conclusion, urine output may predict survival rates of septic AKI patients undergoing CRRT. In these patients, urine output <30 mL/hour during the first 24 hr was a strong risk factor for in-hospital mortality.
Figures and Tables
ACKNOWLEDGEMENTS
This study was financially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2058250).
References
1. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644–1655.

2. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995; 273:117–123.

3. Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000; 28:3405–3411.

4. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–1310.

5. Gerlach H, Toussaint S. Sepsis therapy--Why change-management of sepsis can lower its lethality. Anasthesiol Intensivmed Notfallmed Schmerzther. 2006; 41:614–624.
6. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003; 348:1546–1554.

7. Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. CUB-Réa Network. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003; 168:165–172.
8. Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996; 24:192–198.

9. Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002; 30:2051–2058.

10. Druml W. Acute renal failure is not a "cute" renal failure. Intensive Care Med. 2004; 30:1886–1890.

11. Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005; 21:239–249.

12. Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996; 11:293–299.

13. Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007; 33:409–413.

14. Section 2: AKI Definition. Kidney Int Suppl (2011). 2012; 2:19–36.
15. Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007; 18:1292–1298.

16. Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009; 37:2552–2558.

17. Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009; 35:1692–1702.

18. Mandelbaum T, Lee J, Scott DJ, Mark RG, Malhotra A, Howell MD, et al. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med. 2013; 39:414–419.

19. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008; 73:1008–1016.

20. Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014; 29:1301–1311.

21. Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015; 10:147–155.

23. Wald R, Deshpande R, Bell CM, Bargman JM. Survival to discharge among patients treated with continuous renal replacement therapy. Hemodial Int. 2006; 10:82–87.

24. Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med. 2009; 37:2576–2582.

25. Kawarazaki H, Uchino S, Tokuhira N, Ohnuma T, Namba Y, Katayama S, et al. Who may not benefit from continuous renal replacement therapy in acute kidney injury? Hemodial Int. 2013; 17:624–632.

26. Wu VC, Ko WJ, Chang HW, Chen YW, Lin YF, Shiao CC, et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med. 2008; 34:101–108.

27. Ostermann M, Chang RW. Correlation between parameters at initiation of renal replacement therapy and outcome in patients with acute kidney injury. Crit Care. 2009; 13:R175.

28. Cerdá J, Cerdá M, Kilcullen P, Prendergast J. In severe acute kidney injury, a higher serum creatinine is paradoxically associated with better patient survival. Nephrol Dial Transplant. 2007; 22:2781–2784.

29. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009; 20:2305–2313.

30. Mehta RL. Acute kidney injury: Urine output in AKI--the canary in the coal mine? Nat Rev Nephrol. 2013; 9:568–570.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download