The existence of two growth phases is scientifically accepted for cutaneous melanoma (M): the radial growth phase (RGP) and the vertical growth phase (VGP).123 The latter is burdened by a metastatic potential directly correlated to the depth of invasion.4 Depth of invasion was first reported as a prognostic predictor in primary M of the skin by the pathologist Alexander Breslow in 1970.5 Previously, Wallace H. Clark developed a staging system, based on the cutaneous levels of anatomical involvement (level I: epidermis; level II: papillary dermis; level III: adventitial dermis; level IV: reticular dermis; level V: hypodermis).6 Subsequent studies confirmed and refined the role of the depth of invasion in the prognosis of malignant M and showed that Clark's level, although still justifiably adopted, has a lower predictive value, is less reproducible, and is more operator-dependent as compared with Breslow's depth.7 Currently, Breslow's depth is included in the American Joint Committee on Cancer (AJCC) staging system for M as a major prognostic determinant.48 The approximate 5-year survival is 95-100% for thin M (≤1 mm), and its prognostic variability can be explained by the existence of an early (<1 mm) VGP.3910 On the other hand, the approximate 5-year survival ranges from 50 to 96% for thick M (>1 mm): in detail 80-96% for 1.1-2.0 mm, 60-75% for 2.1-4.0 mm, 50% >4 mm.8 However, in its original work, Breslow identified five stages according to the thickness, as follows: stage I (≤0.75 mm), stage II (0.76-1.50 mm), stage III (1.51-2.25 mm), stage IV (2.26-3.00 mm), stage V (≥3.00 mm).5 Therefore, the author recognized in ‘3 mm’ a critical measure for VGP.7 At this depth, the survival chances are more or less equal to 50% and the biological behavior follows the ‘all-or-none law’, where ‘all’ refers to a rapid fatal evolution, due to widespread dissemination, and ‘none’ stands for 5-year survival free of disease. Thanks to this extraordinary forensic case, we have reached the conclusion that the ‘all-or-none law’ applied to VGP of cutaneous malignant M appears to be proportionally related to mitogenicity, despite of free resection margins and not evident lympho-vascular invasion. In fact, the absence of lympho-vascular invasion in the surgical specimen does not exclude an occult involvement in transit. Briefly, in 1983 a 25-mm-width 3-mm-thick verrucous nevoid M with minimal deviation (3 mitoses per mm2 on average) arose in the left thigh of a 37-year-old woman and it was misdiagnosed for verrucous nevus, as unfortunately it sometimes occurs even today due to the high degree of diagnostic difficulty.11 This diagnostic error often has devastating consequences because nevoid M is a VGP M.11 After exactly 5 years (1988), the neoplasia recurred at the same site in the chameleonic form of a signet-ring cell M, another M histotype in VGP by definition,12 and it was misdiagnosed as balloon cell nevus (Fig. 1). The finding of a signet-ring appearance in malignant M is an unusual event, being observed in only the 0.5% of cases, and it reflects metastatic or recurrent lesions.12 After another 5 years (1993), the neoplasia recurred at the same site and a diagnosis of malignant VGP M with nevoid aspects was formulated, because the neoplasia recurred with the original features. In 1999, the patient developed metastasis to regional nodes, and in 2008 she died from diffuse metastasization. In the described case, the Breslow's depth exactly coincided with the above-mentioned 3 mm, but the patient died many years (25 years) after the initial misdiagnosis. Moreover, until its final dissemination, the tumor showed a regular recurrence time of 5 years despite of its histological appearance; this datum seems to exclude a different prognostic impact for the two reported histotypes, which have VGP as their common prognostic factor. In light of this, the ‘all-or-none law’ applied to advanced VGP appears to be mitigated through time by low mitogenicity, as can be encountered in the unusual variants of malignant M with minimal deviation.
Figures and Tables
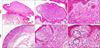 | FIG. 1Original slides dating back to 1983: the nevoid melanoma showed a verrucous growth pattern at scanning magnification (A, haematoxylin/eosin, ×1.25), peripheral densely cellular nodules with epidermal thinning at higher magnification (B, haematoxylin/eosin, ×10), and some mitotic figures pointed out by black arrows (C, haematoxylin/eosin, ×40). Original slides dating back to 1988: the signet-ring cell melanoma did not show a verrucous appearance (D, haematoxylin/eosin, ×4), it consisted of clear atypical cells (E, haematoxylin/eosin, ×20) with a signet-ring (F, arrows) or physalipherous (F, circles) morphology (F, haematoxylin/eosin, ×40). |
References
1. Roncati L, Piscioli F, Pusiol T. Clinical application of the unifying concept of cutaneous melanoma. Chonnam Med J. 2017; 53:78–80.

3. Lefevre M, Vergier B, Balme B, Thiebault R, Delaunay M, Thomas L, et al. Relevance of vertical growth pattern in thin level II cutaneous superficial spreading melanomas. Am J Surg Pathol. 2003; 27:717–724.

4. Gershenwald JE, Scolyer RA, Hess KR, Thompson JF, Long GV, Ross MI, et al. Melanoma of the skin. In : Amin MB, editor. AJCC cancer staging manual. 8th ed. Chicago: Springer;2017. p. 563–585.
5. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970; 172:902–908.

6. Clark WH Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969; 29:705–727.
7. Büttner P, Garbe C, Bertz J, Burg G, d'Hoedt B, Drepper H, et al. Primary cutaneous melanoma. Optimized cutoff points of tumor thickness and importance of Clark's level for prognostic classification. Cancer. 1995; 75:2499–2506.

8. Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001; 19:3622–3634.

9. Roncati L, Pusiol T, Piscioli F. Thin melanoma: a generic term including four histological subtypes of cutaneous melanoma. Acta Dermatovenerol Croat. 2016; 24:169–174.
10. Piscioli F, Pusiol T, Roncati L. Histopathological determination of thin melanomas at risk for metastasis. Melanoma Res. 2016; 26:635.

11. Bastian BC, Lazar A. Nevoid melanoma. In : Calonje E, Brenn T, Lazar A, McKee PH, editors. McKee's pathology of the skin: with clinical correlations. 4th ed. Edinburgh: Elsevier Saunders;2012. p. 1240.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download