Abstract
Delayed arterial healing at culprit sites after drug-eluting stent (DES) placement for acute myocardial infarction (AMI) is associated with increased risk of late stent thrombosis. The Korea Acute Myocardial Infarction Registry was established in commemoration of the 50th anniversary of Korea Circulation Society. Between November 2005 and December 2016, more than 62,000 patients were registered from 50 primary percutaneous coronary intervention (PCI) centers in Korea. DES in AMI may be safe and effective, however, there is concern about increased stent thrombosis after DES implantation in AMI patients, requiring longer-term dual anti-platelet therapy to reduce the risk of late stent thrombosis. Device innovation is needed to overcome issues such as stent thrombosis and restenosis by using new coating materials with biocompatible polymers, different coating methods using non-polymer techniques, bioabsorbable stents and pro-healing stents. In this review article, we describe the current usage of DES in AMI in Korea and introduce novel DES uses in development for patients with AMI. We have developed many types of DES in our research laboratory. Abciximab-coated stents inhibited platelet thrombi and restenosis. Furthermore, anti-oxidants (carvedilol, probucol and alpha-lipoic acid) were used for stent coating. Currently we are developing novel DESs using polymer-free and natural binding techniques, peptide coating stents, gene-and-drug delivery, bioabsorbable stents using 3D printing, endothelial progenitor cell capturing stents to promote reendothelialization and reduce stent thrombosis. New DESs in development may be safe and effective in preventing late stent thrombosis and restenosis in patients with AMI.
The main characteristics of the culprit lesions in acute myocardial infarction (AMI) include a lower plaque volume with a higher thrombus burden. Healing patterns of the target lesions in AMI are different from those with stable angina. Delayed arterial healing at the culprit sites after drug-eluting stent (DES) placement for AMI is associated with increased risk of late stent thrombosis.
In the present review article, we describe the current use of DES in AMI in Korea and introduce novel DES uses in development for patients with AMI.
The Korea Acute Myocardial Infarction Registry (KAMIR) was established in commemoration of the 50th anniversary of Korea Circulation Society.1 We have registered more than 62,000 patients with AMI since 2005. Rates of percutaneous coronary intervention (PCI) were 96.7% in ST-elevation myocardial infarction (STEMI) and 82.7% in non-ST-elevation myocardial infarction (NSTEMI), and the success rates were 99.4% and 99.5%, respectively.2 Penetration rate of DES is higher than 90% (97% currently) in Korea.3 Stent thrombosis occurred in 1.1 % of patents after implantation of Cypher® (Cordis Corporation, Bridgewater, NJ, USA) and Taxus® (Boston Scientific, Natick, MA, USA) stents during a 6-month clinical follow-up.4 The outcome of DES was compared with that of bare metal stents (BMS) in patients with AMI. The event rates were lower in patients receiving DES compared to BMS with lower incidence of repeat intervention without increasing the risk of mortality, myocardial infarction (MI) and stent thrombosis.5 However, safety issues of DES still need to be addressed in real-world practice. Based on our clinical study using intravascular ultrasound (IVUS), we found that plaque prolapse occurred frequently in as many as 27% of patients with AMI after stent implantation. Predictors of plaque prolapse were long stent length and ruptured plaques, indicating plaque prolapse may be one of the risk factors for stent thrombosis in AMI.6 Virtual histology-IVUS examination in diabetic AMI patients showed multiple plaque rupture and 72% of thrombus in 60% and 72% of cases, respectively.7 No reflow developed in approximately 10 to 20% of AMI patients after stent implantation. IVUS predictors of no reflow after stenting were high density calcium and large necrotic cores at minimal luminal sites.8 High degrees of calcification and multiple plaque rupture were demonstrated in AMI patients with chronic kidney disease.9 Clinical, angiographic and IVUS predictors of early stent thrombosis were analyzed in patients with AMI. Tissue prolapse, high level of troponin and no reflow phenomenon were associated with early stent thrombosis in AMI after stent implantation.10
Current limitations of DES are development of late stent thrombosis and inflammation mediated by polymer-degradation.11 The cause of stent thrombosis after DES implantation is multifactorial, incomplete endothelial coverage being the most powerful histologic predictor in combination with other clinical and procedural risk factors playing a role.1213 We have developed new DES to prevent stent thrombosis and restenosis.14 We established the Korea Cardiovascular Stent Research Institute and Mangho Stent Company. We have developed the Chonnam National University Hospital (CNUH) stent, a coronary stent with a design of our own.15 The CNUH stent was more flexible than four commercialized stents tested which are the Promus Element® (Boston Scientific, Natick, MA, USA), Cypher® (Cordis Corporation, Bridgewater, NJ, USA), Resolute Integrity® (Medtronic, Santa Rosa, CA, USA), Xience Prime® (Abbott Vascular, Santa Clara, CA, USA) stents.15
Heparin-coated stents inhibited stent restenosis and thrombosis in a porcine model. However, their clinical benefits could not be demonstrated.16 Platelet glycoprotein IIb/IIIa receptor blocker (ReoPro®)-coated stents inhibited platelet thrombi and restenosis in a porcine model and were associated with lower inflammation compared with Cypher® and Taxus® stents.17 ReoPro®-coated stents were feasible and significantly reduced in-stent neointimal hyperplasia in human coronary arteries, with potential therapeutic benefit in preventing stent restenosis (Fig. 1).18 Furthermore, ReoPro®-coated stent implantation was safe, and prevented stent thrombosis and neointimal hyperplasia on follow-up coronary angiograms and IVUS study in AMI patients.19 Target lesion revascularization and total major adverse cardiac events were lower in AMI patients who received ReoPro®-coated stents, compared with BMS.
Anti-oxidants [carvedilol, probucol and alpha-lipoic acid (ALA)], were used for stent coating.202122 In a porcine model, carvedilol stents were more effective than probucol stents.21 No stent thrombosis was observed in clinical trials using carvedilol stents during a two-year clinical follow-up.23 Recently, we developed a ALA-coating stent, which inhibited neointimal cell proliferation by inhibiting phosphorylated extracellular signal-regulated kinases and phosphorylated signal transducers and activators of transcription 3, and promoted endothelial coverage in vitro study.22 ALA-coated stents inhibited Akt and vascular endothelial growth factor expression in a porcine model and may be effective for AMI patients with diabetes mellitus (Fig. 2).22
Non-polymer coating technology was developed to prevent stent thrombosis. New DES was developed using a titanium dioxide (TiO2) film by plasma enhanced chemical vapor deposition as a drug-combining matrix.24 Abciximab, ALA and heparin were effectively coated on the TiO2 film-coated stent surface, which was assessed by atomic focus microscopic imaging studies and still observed even after a one-month washing test. This non-polymer coating technique was registered as US patent in 2015 (US patent: 8,999,456B2).
A dual-coating DES by grafting ALA with Abciximab was prepared on a BMS using non-polymer coating technology.25 Polymer-free TiO2 stent with abciximab or ALA was compared with biolimus-eluting stent (BES) in a porcine model. Percent area stenosis was not different between TiO2 stents with Abciximab or ALA and BES. However, fibrin and inflammation scores were lower in Abciximab and ALA-coated stents than in BES.26 We introduced nitrogen-doped TiO2 film as drug-binding matrices for the preparation of DES. Scanning electron microscope (SEM) findings revealed inhibition of platelet adhesion on heparin-, ALA- and Abciximab-grafted stent surfaces. Abciximab, heparin and ALA were released slowly from the stent surface over 1 month on released kinetics.27 Polymer-free DESs coated with everolimus using nitrogen-doped TiO2 film deposition were tested in vitro and in vivo in a porcine restenosis model.28 Electro-spinning coating with TiO2 film was used and this coating technique showed a rate of cumulative drug release to that of bioabsorbable polymer [poly lactic-co-glycolic acid (PLGA)] coating on the release kinetics. Coating thickness of everolimus-coated TiO2 film was 50 nm, which is the thinnest coating technology so far developed (Fig. 3). In an animal study, percent area stenosis of everolimus nitrogen doped TiO2 stent was comparable with Xience Prime® stents but the fibrin score was significantly lower in everolimus nitrogen doped TiO2 stents than Xience Prime® stents (Fig. 4).
Dextran is a natural polymers. An in vitro and in vivo comparison of dextran-based sirolimu-eluting stents and polylactic acid-based sirolimus stents was performed.29 The contact angle and the inflammation score of dextran-based DESs were lower than those of polylactic acid-based DESs. Immunofluorescence analysis revealed enhanced re-endothelialization and reduced inflammation in the dextran-based DES.
Biocompatible co-polymer systems using PLGA-polyethylene glycol (PEG) co-polymer coronary stents were evaluated in a porcine model.30 Novel coronary artery stents with co-polymers showed great potential for future clinical applications and may be applied in the development of novel coronary artery stents with biodegradable polymers.
Tacrolimus-eluting stents using a biodegradable polymer-coating technique were compared with sirolimus- and everolimus-eluting stents.31 The CNUH stents were used for the stent platform of 3 types of DESs. Inhibitory effects on smooth muscle cells (SMC) and human umbilical vein endothelial (HUVEC) proliferation were dose-dependent in all the 3 types of DESs.
The effect of a novel peptide, WKYMVm- and sirolimus-coated stents on re-endothelialization and anti-restenosis was assessed in an animal model.32 The WKYMVm peptide, specially synthesized for homing endothelial colony-forming cells, was coated onto a BMS with hyaluronic acid. Thereafter, sirolimus was consecutively coated. The peptide was effectively attached to the surface of the stents and the sirolimus coating rendered the surface smooth. The release pattern of sirolimus was similar to that of commercial sirolimus-coated stents. Endothelial-cell proliferation was enhanced, whereas the proliferation of SMC was inhibited. In an animal study, the restenosis rates were lower than those of BMS and the endothelial healing was promoted, suggesting that consecutive coatings of WKYMVm and sirolimus onto BMS may have a potential role in re-endothelialization and neointimal suppression (Fig. 5).32 This innovation was registered as a US patent (US Patent: 9603891).
Endothelial progenitor cell (EPC)-capturing Aptamer stents improved re-endothelialization compared with BMS and Taxus® Stent.33 EPC-capturing Aptamer stents showed lower re-endothelialization scores and thrombus appearance rates compared to Taxus® stent.
A natural binding technique was applied on the stent surface. A natural product, fucoidan, was used as a coating material onto BMS with in vivo evaluation. Fucoidan is derived from sea tangle and has anti-oxidant and mitogen-associated kinase inhibitory activities. Three coating technologies, multi-layer, single-layer and dual coating methods, were tested in Korea Cardiovascular Research Institute. Dual-coating stents with PLGA in the outer coating and fucoidan in the inner coating layers reduced percent area stenosis.34
Dopamine is a major component of mussels. Thromboresistant and endothelialization effects of dopamine-mediated heparin coating on a stent material surface were tested.35 Dopamine-mediated heparin coating improved endothelialization and inhibited platelet deposition. This innovation was registered as a Japanese patent (Japan patent: 5576441).
Phytoncide, a natural product derived from trees and plants was utilized for the coating of DES.36 Phytoncide-coated stents compared favorably with sirolimus eluting stents in terms of neointimal area, inflammation and fibrin scores.
A novel DES with a dual therapy system was developed consisting of an abluminal and luminal coating technique.37 The abluminal coating layer contains anti-proliferative agent such as sirolimus, everolimus, paclitaxel and the luminal coating layer contains a re-endothelialization agent such as the WPP peptide or the CD34 antibody. In order to verify the bidirectional coating of materials, various morphologic analyses were performed using optical microscopy, SEM and fluorescence microscopy. The proliferation of SMC was inhibited by sirolimus, whereas the proliferation of HUVEC cells was enhanced by WKYMVm.
Gene delivery stents using TiO2 and drug coating were developed.38 Plasmids were attached on the TiO2 film drug-coated stent surface and gene delivery was demonstrated in the porcine coronary artery SMC using stents and in the rat abdominal wall using gene-coated plate. a follow-up coronary angiogram revealed a patent stent without restenosis and with a clean surface of the stented artery. Histopathologic findings showed a small area of neotintima formation (Fig. 6).38 This innovation was registered as a Japanese patent in 2016 (Japan patent: 5922647). Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent was tested in a rabbit model.39 On micro-computed tomography scans, the neointimal area was smaller in Akt1 siRNA-stents than those of BMS and hyaluronic acid-coated stents.
A bio-absorbable drug-coated stent was developed using a 3D-printing system with characterization and in vivo evaluation (Fig. 7).40 The fabricated polycaprolactone stent was coated with sirolimus mixed with PLGA and PEG via a spraying method for slow drug release. The engineered, drug-eluting, bioabsorbable vascular stent (BVS) proved effective in reducing neointimal hyperplasia in an animal study (Fig. 8).40
New BVSs using magnesium were developed and a smart stent with a micro-sensor for coronary pressure measurements is in development. Femtosecond laser stents are one of the new concepts of non-polymeric drug coating system, where femtosecond laser holes may increase biocompatibility and regulate cell proliferation with alignment.
The present study described experiences from a single center on novel DESs, and thus may be limited in scope to reflect the real-world status of DES development. In addition, a number of DES models including stents with anti-oxidants, natural binding techniques, and gene-and-drug delivery capabilities are investigational and have yet to receive regulatory approval for clinical trials in Korea.
In conclusion, DESs are deemed safe and effective for the prevention of restenosis. However, problems such as late or very late stent thrombosis and late catch-up remain be to be solved. Currently, longer-term, dual anti-platelet therapy is recommended after DES implantation, especially in patients with AMI. Novel DES using non-polymer coating, natural binding technology, peptide coating, gene-and-drug delivery as well as bioabsorbable stents with new biology may improve long-term clinical outcomes and shorten the duration of dual antiplatelet therapy in AMI patients.
Figures and Tables
FIG. 1
Scanning electron microscopic findings after abciximab coating on the surface of the stent. [Fig. 1 of 18].

FIG. 2
Methyl methacrylate stain in the α-lipoic acid coated stent study. In-stent neointimal area was smaller in the α-lipoic acid coated stent group (B) compared with the control group (A). Magnification: 10×. [Modified from Fig. 4 of 22].

FIG. 3
Scanning electron microscopy images of the stent surface. Images of the stent according to longitudinal direction were represented (A). The coating thickness was measured in cross sectional view (B). [Fig. 2 of 28].

FIG. 4
Histomorphometric analysis of polymer-free DES in porcine coronary arteries. Area stenosis (A) and fibrin score (B). *p<0.05, **p<0.01, ***p<0.001. Group 1: BMS, group 2: BMS with NTiO2, group 3: durable-polymer EES, and group 4: polymer-free EES using NTiO2. [Modified from Fig. 4 of 28]. BMS: bare-metal stent, DES: drug-eluting stent, EES: everolimus-eluting stent, NTiO2: nitrogen-doped titanium dioxide.

FIG. 5
Cross sections of rabbit iliac arteries 6 weeks after stent implantation. Immunohistochemistry for investigating re-endothelialization was performed using a CD31 antibody. CD31 staining was incomplete in BMS and durable-polymer EES (Xience Prime™) groups, whereas CD31 stained with a consecutive linear pattern in the HA-Pep and Pep/SRL groups, suggesting peptide coating promotes endothelialization. [Modified from Fig. 6 of 32]. BMS: bare-metal stent, EES: everolimus-eluting stent, HA-Pep: hyaluronic acid-peptide, Pep/SRL: sirolimus coated onto HA-Pep, SRL: sirolimus.

FIG. 6
Binding plasmid (gWIZ-beta-gal) was effectively transfected into 293 cells after detachment (Fig. 1A). Five days after implantation of TiO2-abciximab-beta-gal plasmid stainless steel plates in rat abdomen and implantation of TiO2-abciximab-beta-gal plasmid bound to a pig coronary stent, the expression of beta-gal plasmid was observed (Fig. 1B). To analyze the inhibitory effect on ISR, we used both TiO2-abciximab-KLF4-plasmid-cobalt chromium stent [TAK-CC, Fig. 1C (right)] and TiO2-only cobalt chromium stent [T-CC, Fig. 1C (left)]. Four weeks after implantation in porcine coronary artery, by histomorphometric analysis, the TAK-CC group showed decreased ISR (Fig. 1C). [Modified from Fig. 1. of 38]. ISR: in-stent restenosis, KLF4: Kruppel-like factor 4, TAK-CC: TiO2-abciximab-KLF4-plasmid-cobalt chromium stent, T-CC: TiO2-only cobalt chromium stent, TiO2: Titanium dioxide.
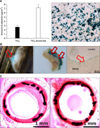
FIG. 7
Schematic design of a 3D-printing system (A), an ultrasonic-spray coating system (B), an optical microscope image of a BVS fabricated by a 3D-printing system (C), and a weight changes (D). [Modified from Fig. 1 of 40]. BVS: bioabsorbable vascular scaffold.

FIG. 8
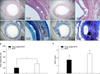
Images of hematoxylin-eosin and Carstair fibrin staining after 4 weeks of drug-coated BVS (A and C) and BVS (B and D). Area restenosis (%) (E), and fibrin score (F). *p<0.05. Sirolimus-coated BVS was effective in reducing neointimal hyperplasia compared with non-coated BVS at 4 weeks after stent implantation (E). Fibrin score was higher in the drug-coated BVS group (F). [Modified from Fig. 3 of 40]. BVS: bioabsorbable vascular scaffold.
ACKNOWLEDGMENTS
This study was supported by grants from the Korean Health Technology R&D Project (HI13C1527), Ministry of Health & Welfare, Republic of Korea.
References
1. Lee KH, Jeong MH, Kim HM, Ahn Y, Kim JH, Chae SC, et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2011; 58:1664–1671.

2. Kim JH, Chae SC, Oh DJ, Kim HS, Kim YJ, Ahn Y, et al. Multicenter cohort study of acute myocardial infarction in Korea - Interim analysis of the Korea acute myocardial infarction registry-national institutes of health registry. Circ J. 2016; 80:1427–1436.

3. Kook HY, Jeong MH, Oh S, Yoo SH, Kim EJ, Ahn Y, et al. Current trend of acute myocardial infarction in Korea (from the Korea Acute Myocardial Infarction Registry from 2006 to 2013). Am J Cardiol. 2014; 114:1817–1822.

4. Lee SR, Jeong MH, Ahn YK, Chae SC, Hur SH, Kim YJ, et al. Clinical safety of drug-eluting stents in the Korea acute myocardial infarction registry. Circ J. 2008; 72:392–398.

5. Hong YJ, Jeong MH, Ahn Y, Kang JC. The efficacy and safety of drug-eluting stents in patients with acute myocardial infarction: results from Korea Acute Myocardial Infarction (KAMIR). Int J Cardiol. 2013; 163:1–4.

6. Hong YJ, Jeong MH, Ahn Y, Sim DS, Chung JW, Cho JS, et al. Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging. 2008; 1:489–497.

7. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging. 2009; 2:339–349.

8. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011; 32:2059–2066.

9. Hong YJ, Jeong MH, Choi YH, Ma EH, Ko JS, Lee MG, et al. Effect of renal function on ultrasonic coronary plaque characteristics in patients with acute myocardial infarction. Am J Cardiol. 2010; 105:936–942.

10. Hong YJ, Jeong MH, Choi YH, Park SY, Rhew SH, Jeong HC, et al. Clinical, angiographic, and intravascular ultrasound predictors of early stent thrombosis in patients with acute myocardial infarction. Int J Cardiol. 2013; 168:1674–1675.

11. Lim SY, Jeong MH, Hong SJ, Lim DS, Moon JY, Hong YJ, et al. Inflammation and delayed endothelization with overlapping drug-eluting stents in a porcine model of in-stent restenosis. Circ J. 2008; 72:463–468.

12. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007; 115:2435–2441.

13. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202.
14. Cho JY, Ahn Y, Jeong MH. A bumpy and winding but right path to domestic drug-eluting coronary stents. Korean Circ J. 2013; 43:645–654.

15. Bae IH, Lim KS, Park JK, Park DS, Lee SY, Jang EJ, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015; 21:1295–1300.

16. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Cho JG, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999; 48:324–330.

17. Hong YJ, Jeong MH, Lee SR, Hong SN, Kim KH, Park HW, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007; 22:802–809.

18. Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004; 94:1050–1054.

19. Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: ReoPro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006; 47:933–938.

20. Kim W, Jeong MH, Cha KS, Lee SH, Lim JH, Kim HG, et al. The effect of the probucol-loaded biodivYsioTM DD stent on inhibition of neointimal proliferation in porcine coronary stent restenosis model. Korean Circ J. 2003; 33:1028–1035.

21. Kim W, Jeong MH, Cha KS, Hyun DW, Hur SH, Kim KB, et al. Effect of anti-oxidant (carvedilol and probucol) loaded stents in a porcine coronary restenosis model. Circ J. 2005; 69:101–106.

22. Lim SY, Bae EH, Jeong MH, Kim JH, Hong YJ, Sim DS, et al. Effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009; 54:375–385.
23. Kim HK, Hong YJ, Jeong MH, Kim W, Kim SS, Ko JS, et al. Two-year clinical outcome after carvedilol-loaded stent implantation in patients with coronary artery disease. Korean J Intern Med. 2011; 26:41–46.

24. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2010; 20:4792–4801.

25. Song SJ, Kim KS, Park YJ, Jeong MH, Ko YM, Cho DL. Preparation of a dual-drug-eluting stent by grafting of ALA with abciximab on a bare metal stent. J Mater Chem. 2009; 19:8135–8141.

26. Lim KS, Jeong MH, Bae IH, Park JK, Park DS, Kim JM, et al. Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model. J Cardiol. 2014; 64:409–418.

27. Song SJ, Jung KW, Park YJ, Park J, Cho MD, Jeong MH, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparation of drug-eluting stents. J Mater Chem. 2011; 21:8169–8177.

28. Sim DS, Jeong MH, Park DS, Kim JH, Lim KS, Bae IH, et al. A novel polymer-free drug-eluting stent coated with everolimus using nitrogen-doped titanium dioxide film deposition in a porcine coronary restenosis model. Int J Cardiol. 2016; 222:436–440.

29. Lee SY, Bae IH, Park DS, Jang EJ, Shim JW, Lim KS, et al. Comparison of dextran-based sirolimus-eluting stents and PLA-based sirolimus-eluting stents in vitro and in vivo. J Biomed Mater Res A. 2017; 105:301–310.

30. Lim KS, Park JK, Jeong MH, Hah JW, Kim DG, Bae IH, et al. Comparison of sirolimus loaded PLGA-PEG Co-polymer coronary stent and bare metal stent in a porcine coronary restenosis model. Macromol Res. 2014; 22:639–646.

31. Park DS, Park JK, Jeong MH, Bae IH, Lee SY, Jang EJ, et al. Tacrolimus-eluting stent with biodegradable polymer is more effective than sirolimus-and everolimus-eluting stent in rabbit iliac artery restenosis model. Macromol Res. 2015; 23:1034–1041.

32. Jang EJ, Bae IH, Park DS, Lee SY, Lim KS, Park JK, et al. Effect of a novel peptide, WKYMVm- and sirolimus-coated stent on re-endothelialization and anti-restenosis. J Mater Sci Mater Med. 2015; 26:251.

33. Sim DS, Kwon JS, Kim YS, Chung HC, Hong YJ, Park HW, et al. Experience with endothelial progenitor cell capturing aptamers for coating of intracoronary stents in a porcine model. Tissue Eng Regen Med. 2009; 6:555–561.
34. Kim JM, Bae IH, Lim KS, Park JK, Park DS, Lee SY, et al. A method for coating fucoidan onto bare metal stent and in vivo evaluation. Prog Org Coat. 2015; 78:348–356.

35. Bae IH, Park IK, Park DS, Lee H, Jeong MH. Thromboresistant and endothelialization effects of dopamine-mediated heparin coating on a stent material surface. J Mater Sci Mater Med. 2012; 23:1259–1269.

36. Kang SN, Kim SE, Choi J, Park K, Goo JH, Sim DS, et al. Comparison of phytoncide with sirolimus as a novel drug candidate for drug-eluting stent. Biomaterials. 2015; 44:1–10.

37. Park JK, Lee JH, Nah JW, Kim HK, Lim KS, Bae IH, et al. Development of a novel drug-eluting stent consisting of an abluminal and luminal coating layer dual therapy system. RSC Adv. 2015; 5:40700–40707.

38. Kwon JS, Song SJ, Yang EJ, Kim YS, Lim KS, Kim DG, et al. Novel abciximab-Kruppel-like factor 4-plasmid dual-delivery titanium dioxide-coated coronary stent. Int J Cardiol. 2013; 168:5104–5106.

39. Che HL, Bae IH, Lim KS, Song IT, Lee H, Muthiah M, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download