Abstract
Recepteur d'origine nantais (RON) is a receptor tyrosine kinase belonging to the subfamily of which c-MET is the prototype. Large epidemiologic studies have confirmed the strong association between RON and gastric cancer development. Constitutive activation of RON signaling directly correlates with tumorigenic phenotypes of gastric cancer and a poor survival rate in advanced gastric cancer patients. In this review, we focus on recent evidence of the aberrant expression and activation of RON in gastric cancer tumors and provide insights into the mechanism of RON signaling associated with gastric cancer progression and metastasis. Current therapeutics against RON in gastric cancer are summarized.
Recepteur D'origine Nantais (RON), a protein encoded by the MST1R gene in humans, is a receptor tyrosine kinase (RTK) that belongs to the subfamily of which c-MET is the prototype. It was first discovered in humans as a novel protein sharing 34% and 63% amino acid sequence identity with c-MET in extracellular and cytoplasmic kinase domains, respectively (Fig. 1).1 Human RON is a disulfide-linked heterodimer protein consisting of an approximately 40-kDa completely extracellular α-chain and an approximately 150-kDa single-pass transmembrane β-chain.2 The extracellular domain is responsible for ligand binding, receptor dimerization, and phosphorylation. This domain is divided into semaphoring (Sema), plexin semaphorin-integrin (PSI), and three immunoglobulin-plexin-transcription factor (IPT1-IPT3) subdomains.3 The intracellular portion has TK catalytic sites (Tyr 1238 and Tyr 1239) and carboxy-terminal tail (Tyr 1353 and Tyr 1360) act as docking sites for the recruitment of transducers and adaptors.4
RON is constitutively transcribed and expressed in different types of cells, mainly of epithelial origins, in the liver, lung, gut, kidney, brain, bone, adrenal gland, and skin.5 Two RON transcripts encoding a full-length RON and a short-form RON (SF RON) are often observed in normal and cancerous tissues.12 The classical RON promoter upstream of the major initiation site is responsible for the initiation of the full-length RON transcript.67 The SF RON transcript encodes an amino terminus-truncated RON that lacks the majority of the extracellular amino acids.18 This isoform is initiated at an alternative initiation site of Met913 from an intragenic promoter between RON introns 8 and 10 (Fig. 2).179 SF RON is constitutively active and present in both normal epithelial and various cancerous and leukemic cells.18 In addition, post-transcriptional modifications of RON, such as alternative splicing, and protein truncation, frequently occur to generate multiple RON isoforms beyond full-length and SF-RON (Fig. 2).1011121314151617 At least nine types of RON isoforms have been identified: RONΔ170, RONΔ165, RONΔ165.e11p, RONΔ160, RONE5/6in, RONΔ155, RONΔp110, RONΔ85 and RONΔ55.1819
The ligand for the RON receptor is the macrophage-stimulating protein (MSP), also termed the hepatocyte growth factor-like protein (HGFL) or the macrophage-stimulating 1 (MST1).2021 The mature and functional MSP encompasses two receptor binding sites that are necessary for ligand-mediated receptor activation.22 MSP stimulation is the major cause of RON activation under physiological conditions.921 However, SF RON is constitutively active in normal cells.189 In tumors, the RON activation is most often due to RON over-expression, RON mutations, generation of splicing variants/truncated forms and, very rarely, increased gene copy numbers (Fig. 3).19
Dimerization of RON in the cell surface is the first step required for RON activation. This activation results in auto-phosphorylation in the kinase domain, leading to further phosphorylation in the C terminal docking site, which recruits cytoplasmic molecules Son of Sevenless (SOS) and the growth factor receptor-bound protein 2 (GRB2).23242526 The negative modulator casitas B-lineage lymphoma (CBL), a ubiquitin ligase, also binds to the docking site, resulting in poly-ubiquitylation of Ron molecules, which are subsequently sorted for endocytosis and degradation (Fig. 3). The docking site interacts with downstream signaling proteins, triggering classical RAS-mitogen-activated protein kinase (MAPK), Janus kinase/signal transducer and activator of transcription 3 (JAK-STAT3), and phosphoinositide 3-kinase-protein kinase B (PI3K-AKT) pathways. These pathways are responsible for increased proliferation, survival, epithelial to mesenchymal transition (EMT), migration, motile-invasive activity, angiogenesis, and chemoresistance (Fig. 4).2728
RON is important in the progression, invasion, and metastasis of gastric carcinoma (Table 1).29 The aberrant expression and activation of RON in gastric tumors as well as gastric cancer cells have been conclusively documented. For examples, RON was expressed in 73% of gastric tumors30 and 56.1% of gastric carcinoma tissues, significantly higher than that in paraneoplastic tissues (25.6%) and completely absent in normal gastric mucosal tissue.31 The latter study also reported that progressively deeper infiltration by gastric carcinoma cells was associated with progressively stronger expression of the RON protein. The protein expression was positively and statistically significantly correlated with perigastric lymph node metastasis and the clinical pathology stage. Its expression was stronger in the Borrmann III/IV group (63.6%) than in the Borrmann I/II group (53.8%), stronger in the distant metastasis group (68.2%) than in the non-distant metastasis group (52.6%), and stronger in the histologically lower differentiated group (52.4%) than in the moderately and highly differentiated histology group (39.1%).31
A study of gastric and gastroesophageal junction tissue samples found that RON was highly over-expressed in 74% of gastroesophageal samples, with over-expression prognostic of poor survival. RON and c-MET co-expression occurred in 43% of samples and were also prognostic of worse survival rates. High RON gene copy numbers were seen in 35.5% of cases that correlated with poor survival. A novel somatic RON juxtamembrane mutation R1018G was also found in 11% of cancer samples.32
A recent study evaluated the expression of RON at RNA and protein levels by RT-PCR and immunohistochemistry in human gastric cancer tissues, paired normal gastric mucosa, and metastatic or non-metastatic lymph node tissues from the same patients acquired by endoscopic biopsy and from surgical specimens.33 The authors confirmed the up-regulation of RON expression in cancer tissues compared with paired normal mucosa at the RNA level. Immunostaining of the RON protein was predominant in the cytoplasm of cancer cells and metastatic lymph node tissues, but was undetectable in the tumor stroma. Patients with positive RON expression reportedly have an elevated risk of death after adjustment for age and tumor size.33
RONΔ165 is a unique RON isoform whose expression has been assessed in gastric cancer tissue. It was first detected as abnormally accumulating in KATO-III human gastric cancer cells. This isoform is constitutively activated by disulfide-linked intracellular oligomerization because it contains an uneven number of cysteine residues. The intracellular activation of this isoform is followed by the acquisition of invasive properties in vitro.10 A subsequent study involving human gastric cancer samples also documented that RONΔ165 was strongly expressed in fresh gastric carcinoma tissue, corresponding paraneoplastic tissue, and perigastric lymph nodes with metastatic carcinoma. In contrast, expression of RONΔ165 was not observed in normal gastric mucosa and normal lymph node tissue samples.31
Aberrant RON expression and activation increase the likelihood of activation of oncogenic signaling leading to the development of gastric cancer. RON small interfering RNA (siRNA) was used to investigate the involvement of RON expression in the development of gastric cancer. Knockdown of RON suppressed gastric tumor cell migration and invasion, and induced cell cycle arrest by decreasing cyclin D1, cyclin D3, and CDK4, and by inducing p21 and p27 expression.33 Phospho-STAT3 (p-STAT3), a poor prognostic indicator for gastric cancer,343536 was determined to be associated with RON and MET expression and activation. p-STAT3 extensity and intensity progressively increased from preneoplastic to neoplastic tissues, and was not detected in adjacent normal tissues. STAT3 was highly expressed in 86% and p-STAT3 in 74% of the tissue samples, respectively, directly correlating with RON and p-RON expression. This data supports the hypothesis that STAT3 is an important downstream mediator of RON and c-MET in gastro-esophageal cancer (Fig. 5).32
It has been shown that signaling cascades, including Akt and MAPK, are significantly blocked by knockdown of RON.33 The authors described that RON expression was significantly associated with tumor size, depth of invasion, lymph node metastasis, tumor stage, and poor survival. These results associated RON expression with gastric tumor progression mediated by the inhibition of cell cycle arrest, enhanced migration, and enhanced cell invasion ability.33 We have demonstrated that RON expression is associated with gastric cancer development through enhanced cancer cell invasiveness. The tumor promoter phorbol 12-myristate 13-acetate (PMA) stimulates RON expression in AGS gastric cancer cells with the main involvement of Egr-1 transcription factors. RON stimulation markedly enhances invasiveness of these cancer cells.37 In another study,38 we revealed that MSP-RON signaling up-regulates the urokinase receptor (UPAR), which in turn enhances cancer cell invasion ability. The UPAR simulation involves the MAPK/extracellular signal-regulated kinase (Erk)-1/2, MAPK/JNK, activator protein-1 (AP-1) and nuclear factor-kappa B (NF-kB) transcription factors (Fig. 5).38
The role of RON expression in apoptotic process of gastric cancer cells has also been investigated. RON targeted siRNA effectively inhibits the constitutive NF-κB activation and alters the ratio of Bax/Bcl-2 in a manner that favors apoptosis. siRNA silencing of RON induces cytochrome c release and the activation of caspase-8 and -9.39 Knockdown of RON leads to an increase in the pre-apoptotic proteins Bid, Bik, BimSL, Bad, and phosphatase and tensin homologs (PTEN), and a decrease in the anti-apoptotic X-linked inhibitor of the apoptosis protein (XIAP) in AGS and MNK28 gastric cancer cells, which ultimately induces apoptosis (Fig. 6). These results indicate the close association of aberrant RON expression and activation with gastric tumor development due to the inhibition of apoptosis and induced growth and mobility of gastric tumor cells.
RON has been clearly implicated in the progression of various cancers, especially pancreatic, colon, breast, and lung cancer.184041 Cancer therapeutic strategies targeting RON that have been developed include hybridoma-based and phage-based monoclonal antibodies, anti-RON antibody to allow drug delivery, small molecular inhibitors, and targeted small peptides.18 Many preclinical studies as well as a clinical trial have been performed to confirm the efficacy of these strategies in treating metastatic diseases, especially pancreatic, colon, and breast cancer.
In gastric cancer, the aberrant expression of RON has been demonstrated, and RON has been implicated in the progression of gastric cancer progression. These findings have identified RON as a potential target for developing new gastric cancer therapies. However, compared to pancreatic, colon, or breast cancer, preclinical and clinical studies of RON targeted therapies for gastric cancer are still limited (Table 2).
IMC-41A10 is a unique RON-specific human antibody developed for gastric cancer therapy by Eli Lilly (ImClone Systems) based on phage-display technology. IMC-41A10 avidly binds to RON and blocks the interaction between MSP and RON. There is no cross-reactivity with c-MET protein and no agonistic activity for RON. The ability of IMC-41A10 to inhibit RON phosphorylation was checked on NIH3T3 cells over-expressing the recombinant wild-type RON protein. When these cells were serum starved and stimulated with MSP, tyrosine phosphorylation of RON was readily detected and could be significantly inhibited (75%) by the addition of IMC-41A10. IMC-41A10 also completely inhibits MSP-induced phosphorylation of MAPK in AGS gastric cancer cells.30
Many of the current small molecular weight tyrosine kinase inhibitors (TKIs) targeting RON have been investigated for their potential in cancer treatment. However, owing to their structural similarities with c-MET and other RTKs, TKIs specific to RON have not been reported.18 Moreover, a study reported the co-expression of c-MET is recognized in 43% of RON-expressing tumors; co-expression of these RTKs was predictive of worse overall survival than over-expression of RON alone.32 Short hairpin RNA (shRNA) knockdown of RON alone in cancer cell lines was found not to prevent tumor progression, while enhancing HGF/c-MET signaling.42 These data suggests that a co-targeted agent for these RTKs is necessary for the successful treatment of cancers is necessary.42
Foretinib (GSK1363089) is an oral multi-kinase inhibitor capable of inhibiting multiple targets, including c-MET, VEGFR-2, RON, and AXL. A preclinical study showed that foretinib effectively inhibits the growth of gastric cancer cells by blocking the signal transduction pathway of tyrosine kinase.43 However, a Phase II clinical study found that this inhibitor did not improve the survival of patients with advanced gastric cancer without previous chemotherapy.44 Therefore, the exact effect of foretinib remains unclear.
T-1840383 is another potent tyrosine kinase inhibitor targeting c-MET, RON, and other TKs, such as VEGFR1-3, RET, RSE, TIE2, and TRKA. T-1840383 inhibited tumor growth in association with reduced p-MET, p-AKT, and p-ERK expression in a MKN45 xenograft model. In a peritoneal dissemination mouse model generated from gastric cancer cells, T-1840383 treatment significantly prolonged survival.45
SU11274 was recognized as a small molecule TKI of c-MET. In gastric cancer, SU11274 can block hepatocyte growth factor-induced EMT, down-regulation of Snail-2 and vimentin, and up-regulation of E-cadherin in MKN45 cells.46 SU11274 can also suppress the proliferation of tumor cells and inhibit their migratory potential. In a mouse model of peritoneal dissemination established from MKN45 cells, SU11274 reduced the numbers and sizes of peritoneal tumors.47 However, a recent study reported that SU11274 inhibits both c-MET signaling and MSP-induced RON signaling.32 This study also confirmed that in AGS cells (Kras mutant, adherent) the inhibition of wound migration was not significant with RON or c-MET protein knockdown alone, but was optimal with the simultaneous knockdown of both proteins. Similarly, apoptosis was highest in the setting of dual c-MET and RON protein knockdown in early and late apoptosis versus either alone. To evaluate the effect of RON and c-MET inhibition combined with chemotherapy, gastro-esophageal cells were treated with SU11274 and/or oxaliplatin chemotherapy, resulting in a synergistic decrease in viability. This suggests the efficacy of SU11274 in gastric cancer treatment; its effect should be mediated by both RON and c-MET signaling impact.
HSP90 was recently implicated as an attractive target for the treatment of cancer because of its central role in oncogenic signaling.484950 Interestingly, evidence exists that RON could be a novel HSP90 client, since mutated RON is susceptible to HSP90 inhibitor-mediated degradation.51 A novel synthetic HSP inhibitor designated EC145 can inhibit RON expression in gastric cancer in physiologically relevant conditions as well as using PMA, which strongly induces RON via protein kinase C signaling.52 Treatment with EC154 reportedly substantially disrupts MSP-RON signaling, as evidenced by diminished downstream phosphorylation and total protein levels of the substrates Erk1/2, Akt, and STAT3 in cancer cells. EC154 leads to an effective dose-dependent inhibition of growth of AGS gastric cancer cells and the growth of gastric tumors in subcutaneous xenograft models of gastric (TMK-I) cancer.
The effectiveness of natural flavonoids against a variety of cancers has been reported.53 We have reported that epigallocatechin gallate (EGCG), the most abundant polyphenol in green tea, can inhibit PMA induced RON expression and reduce RON transcriptional activity in TMK-1, MKN28 and AGS gastric cancer cells.54 Egr-1 transcription factors are down-regulated by EGCG; these factors are important in the EGCG-mediated inhibition of RON expression. EGCG treatment is reported to reduce RON-mediated AGS cell invasion and, in a subcutaneous gastric cancer xenograft model, treatment with EGCG inhibited growth of TMK-1 gastric tumors and substantially decreased in vivo RON expression levels compared to the control group.
Chrysin is another naturally occurring flavonoid with demonstrated anti-cancer effects that are evident as the suppression of RON expression through blockage of Egr-1 and NF-κB in AGS gastric cancer cells, which inhibits gastric cell invasion.55
Recently, we revealed that miR-375 functions as a tumor-suppressor gene in gastric cancer by targeting RON.56 This was the first report of RON regulation mediated by miRNA, which heralds a new strategy for gastric cancer based on RON targeting. The observation that expression of miR-375 reduced transcription activity of 3'-untranslated region (UTR) fragments of RON-encoding mRNA confirmed that miR-375 directly targets the 3'-UTR of RON mRNA. Moreover, we found that over-expression of miR-375 inhibits mRNA and protein expression of RON, accompanied by the suppression of cell proliferation, migration, and invasion in AGS and MKN-28 gastric cancer cells.56 In the same study, ectopic miR-375 expression or knockdown of RON by siRNA induced G1 cell cycle arrest and suppressed tumorigenic properties of cancer cells. This data provides evidence that miR-375 acts as a suppressor of metastasis in gastric cancer by targeting RON, and might represent a new potential therapeutic strategy for gastric cancer treatment.
There is a close association between RON and gastric cancer progression. RON expression is elevated in gastric carcinoma tissue and this expression is correlated with the invasive depth of the tumor and perigastric lymph node metastasis. High RON expression is prognostic of poor survival in advanced gastric cancer patients. The data suggests that controlling RON expression could improve the low efficacy of radiotherapy and chemotherapy that are currently experienced in gastric cancer treatment. The expression and activation of RON may act as a key regulator of gastric cell growth as well as gastric tumor progression. Although current preclinical and clinical studies of anti-gastric cancer therapeutic agents against RON are limited and have mostly involved c-MET targeting agents, a large body of scientific evidence supports the view that blocking RON signaling could offer an advantage toward improving of therapeutic potential in gastric tumors. The collective data indicate that RON expression and activation are important in driving the progression of gastric cancer. Targeting RON is a promising strategy for the treatment of gastric cancer.
Figures and Tables
FIG. 1
Comparison of human RON and c-MET. Sema: semaphoring, PSI: plexin semaphorin-integrin, IPT: immunoglobulin-plexin-transcription factor, TM: transmembrane, TK: tyrosine kinase catalytic sites.

FIG. 3
Receptor activation of RON. SOS: son of sevenless, GRB2: growth factor receptor-bound protein 2, CBL: casitas B-lineage lymphome.
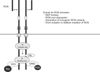
FIG. 4
Downstream signaling of RON activation. GRB2: growth factor receptor-bound protein 2, MAPK: mitogen-activated protein kinases, ERK1/2: extracellular signal-regulated kinase 1/2, IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, EMT: epithelial mesenchymal transition, TGF-β: transforming growth factor beta, NF-κB: nuclear factor kappa light chain enhancer of activated B cells, JAK: janus kinase, STAT3: signal transducer and activator of transcription 3, mTOR: mamalian target of rapamycin, HIF1α: hypoxia inducible factor 1-alplha, P70S6K: ribosomal protein S6 kinase, GSK3β: Glycogen synthase kinase 3 beta.

FIG. 5
Intracellular signals of RON activation for the growth and invasion in gastric cancer cells. CDKI: Cycline-dependent kinase inhibitor, CDK4: Cycline-dependent kinase 4, MAPK: Mitogen-activated protein kinases, Erk1/2: extracellular signal-regulated kinase 1/2, JNK: c-Jun N-terminal kinases, AP-1: Activator protein-1, NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells, UPAR: Urokinase receptor.

FIG. 6
Intracellular signals of RON activation for the cell apoptosis in gastric cancer cells. XIAP: X-linked inhibitor of apoptosis protein, Bcl-2: B-cell lymphoma 2, Bax: Bcl-2-associated X protein, Bik: BCL2 Interacting Killer, Bad: Bcl-2-associated death promoter, Bid: BH3 interacting domain death agonist, BimSL: Bcl-2-like protein 11, PTEN: Phosphatase and tensin homolog.

TABLE 1
Summary of studies investigating the association of RON expression and activation in gastric cancer development

References
1. Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993; 8:1195–1202.
2. Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994; 13:3524–3532.

3. Zarei O, Benvenuti S, Ustun-Alkan F, Hamzeh-Mivehroud M, Dastmalchi S. Strategies of targeting the extracellular domain of RON tyrosine kinase receptor for cancer therapy and drug delivery. J Cancer Res Clin Oncol. 2016; 142:2429–2446.

4. Wang MH, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin. 2010; 31:1181–1188.

5. Quantin B, Schuhbaur B, Gesnel MC, Doll'e P, Breathnach R. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev Dyn. 1995; 204:383–390.

6. Del Gatto F, Gilbert E, Ronsin C, Breathnach R. Structure of the promoter for the human macrophage stimulating protein receptor gene. Biochim Biophys Acta. 1995; 1263:93–95.

7. Angeloni D, Danilkovitch-Miagkova A, Ivanov SV, Breathnach R, Johnson BE, Leonard EJ, et al. Gene structure of the human receptor tyrosine kinase RON and mutation analysis in lung cancer samples. Genes Chromosomes Cancer. 2000; 29:147–156.

8. Bardella C, Costa B, Maggiora P, Patane' S, Olivero M, Ranzani GN, et al. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004; 64:5154–5161.

9. Angeloni D, Danilkovitch-Miagkova A, Ivanova T, Braga E, Zabarovsky E, Lerman MI. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene. 2007; 26:4499–4512.

10. Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996; 16:5518–5526.

11. Santoro MM, Collesi C, Grisendi S, Gaudino G, Comoglio PM. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol Cell Biol. 1996; 16:7072–7083.

12. Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003; 22:186–197.

13. Liu X, Zhao L, Derose YS, Lin YC, Bieniasz M, Eyob H, et al. Short-form ron promotes spontaneous breast cancer metastasis through interaction with phosphoinositide 3-kinase. Genes Cancer. 2011; 2:753–762.

14. Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis. 2000; 21:1507–1512.

15. Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007; 257:157–164.

16. Zhang K, Zhou YQ, Yao HP, Wang MH. Alterations in a defined extracellular region of the RON receptor tyrosine kinase promote RON-mediated motile and invasive phenotypes in epithelial cells. Int J Oncol. 2010; 36:255–264.

17. Ghigna C, De Toledo M, Bonomi S, Valacca C, Gallo S, Apicella M, et al. Pro-metastatic splicing of Ron proto-oncogene mRNA can be reversed: therapeutic potential of bifunctional oligonucleotides and indole derivatives. RNA Biol. 2010; 7:495–503.

18. Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013; 13:466–481.

19. Faham N, Welm AL. RON signaling is a key mediator of tumor progression in many human cancers. Cold Spring Harb Symp Quant Biol. 2016; 81:177–188.

20. Shimamoto A, Kimura T, Matsumoto K, Nakamura T. Hepatocyte growth factor-like protein is identical to macrophage stimulating protein. FEBS Lett. 1993; 333:61–66.

21. Wang MH, Ronsin C, Gesnel MC, Coupey L, Skeel A, Leonard EJ, et al. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994; 266:117–119.

22. Danilkovitch A, Miller M, Leonard EJ. Interaction of macrophage-stimulating protein with its receptor. Residues critical for beta chain binding and evidence for independent alpha chain binding. J Biol Chem. 1999; 274:29937–29943.
23. Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994; 77:261–271.

24. Xiao ZQ, Chen YQ, Wang MH. Requirement of both tyrosine residues 1330 and 1337 in the C-terminal tail of the RON receptor tyrosine kinase for epithelial cell scattering and migration. Biochem Biophys Res Commun. 2000; 267:669–675.

25. Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996; 15:5866–5875.

26. Santoro MM, Penengo L, Minetto M, Orecchia S, Cilli M, Gaudino G. Point mutations in the tyrosine kinase domain release the oncogenic and metastatic potential of the Ron receptor. Oncogene. 1998; 17:741–749.

27. McClaine RJ, Marshall AM, Wagh PK, Waltz SE. Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia. 2010; 12:650–658.

28. Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010; 70:1130–1140.

29. Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011; 34:1774–1780.

30. O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006; 66:9162–9170.
31. Zhou D, Pan G, Zheng C, Zheng J, Yian L, Teng X. Expression of the RON receptor tyrosine kinase and its association with gastric carcinoma versus normal gastric tissues. BMC Cancer. 2008; 8:353.

32. Catenacci DV, Cervantes G, Yala S, Nelson EA, El-Hashani E, Kanteti R, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011; 12:9–46.

33. Song YA, Park YL, Kim KY, Myung E, Chung CY, Cho SB, et al. RON is associated with tumor progression via the inhibition of apoptosis and cell cycle arrest in human gastric cancer. Pathol Int. 2012; 62:127–136.

34. Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS. 2009; 117:598–606.

35. Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, et al. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009; 24:646–651.

36. Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010; 16:5380–5387.

37. Lee KE, Park JS, Khoi PN, Joo YE, Lee YH, Jung YD. Upregulation of recepteur d'origine nantais tyrosine kinase and cell invasiveness via early growth response-1 in gastric cancer cells. J Cell Biochem. 2012; 113:1217–1223.

38. Park JS, Park JH, Khoi PN, Joo YE, Jung YD. MSP-induced RON activation upregulates uPAR expression and cell invasiveness via MAPK, AP-1 and NF-κB signals in gastric cancer cells. Carcinogenesis. 2011; 32:175–181.

39. Park JS, Park JH, Lee S, Joo YE, Jung YD. Small interfering RNA targeting of Recepteur d'Origine Nantais induces apoptosis via modulation of nuclear factor-kappaB and Bcl-2 family in gastric cancer cells. Oncol Rep. 2010; 24:709–714.
40. Kang CM, Babicky ML, Lowy AM. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies. Pancreas. 2014; 43:183–189.

41. Kretschmann KL, Eyob H, Buys SS, Welm AL. The macrophage stimulating protein/Ron pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets. 2010; 11:1157–1168.

42. Zhao S, Cao L, Freeman JW. Knockdown of RON receptor kinase delays but does not prevent tumor progression while enhancing HGF/MET signaling in pancreatic cancer cell lines. Oncogenesis. 2013; 2:e76.

43. Kataoka Y, Mukohara T, Tomioka H, Funakoshi Y, Kiyota N, Fujiwara Y, et al. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs. 2012; 30:1352–1360.

44. Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013; 8:e54014.

45. Awazu Y, Nakamura K, Mizutani A, Kakoi Y, Iwata H, Yamasaki S, et al. A novel inhibitor of c-Met and VEGF receptor tyrosine kinases with a broad spectrum of in vivo antitumor activities. Mol Cancer Ther. 2013; 12:913–924.

46. Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012; 130:2912–2921.

47. Yashiro M, Nishii T, Hasegawa T, Matsuzaki T, Morisaki T, Fukuoka T, et al. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013; 109:2619–2628.

48. Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003; 3:213–217.

49. Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002; 8:4 Suppl. S55–S61.

50. Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl). 2004; 82:488–499.

51. Germano S, Barberis D, Santoro MM, Penengo L, Citri A, Yarden Y, et al. Geldanamycins trigger a novel Ron degradative pathway, hampering oncogenic signaling. J Biol Chem. 2006; 281:21710–21719.

52. Moser C, Lang SA, Hackl C, Zhang H, Lundgren K, Hong V, et al. Oncogenic MST1R activity in pancreatic and gastric cancer represents a valid target of HSP90 inhibitors. Anticancer Res. 2012; 32:427–437.
53. Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001; 84:844–850.

54. Park JS, Khoi PN, Joo YE, Lee YH, Lang SA, Stoeltzing O, et al. EGCG inhibits recepteur d'origine nantais expression by suppressing Egr-1 in gastric cancer cells. Int J Oncol. 2013; 42:1120–1126.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download