Abstract
Melanoma is one of the most aggressive cancers in the world and is responsible for the majority of skin cancer deaths. Recent advances in the field of immunotherapy using active, adoptive, and antigen-specific therapeutic approaches, have generated the expectation that these technologies have the potential to improve the treatment of advanced malignancies, including melanoma. Treatment options for metastatic melanoma patients have been dramatically improved by the FDA approval of new therapeutic agents including vemurafenib, dabrafenib, and sorafenib. These kinase inhibitors have the potential to work in tandem with MEK, PI3K/AKT, and mTOR to inhibit the activity of melanoma inducing BRAF mutations. This review summarizes the effects of the new therapeutic agents against melanoma and the underlying biology of these BRAF inhibitors.
Go to : 
Despite the significant survival rates obtained by surgical excision of primary cutaneous melanomas, metastatic melanoma continues to exhibit frequent relapse and a high mortality rate. Over the last few decades, many therapies have been developed with the goal of improving the survival of metastatic melanoma patients, as current, standard chemotherapy has had a limited impact on the course of this disease. Mutations that confer resistance to targeted therapies, such as the novel protein kinase inhibitors vemurafenib, dabrafenib, and sorafenib, can lead to the simultaneous emergence of resistant clones at many separate body sites, despite an initially positive therapeutic response. This type of relapse may arise within only a few months.1 Approximately 50% of cutaneous melanomas harbor an activating mutation in the gene encoding the protein kinase B-RAF,2 most commonly the B-RAFV600E mutation, a valine to glutamate mutation on codon 600.3 Respectively, 52% and 43% of superficial spreading melanoma and nodular melanoma contain such a mutation. Smaller percentages are seen in acral lentigious and lentigo maligna melanoma, and the frequency of all types varies with the extent of UV exposure.3 Due to its contribution to tumorigenesis, as illustrated above, there has been a significant focus on BRAF-targeted clinical therapy for treatment of metastatic melanoma in patients.
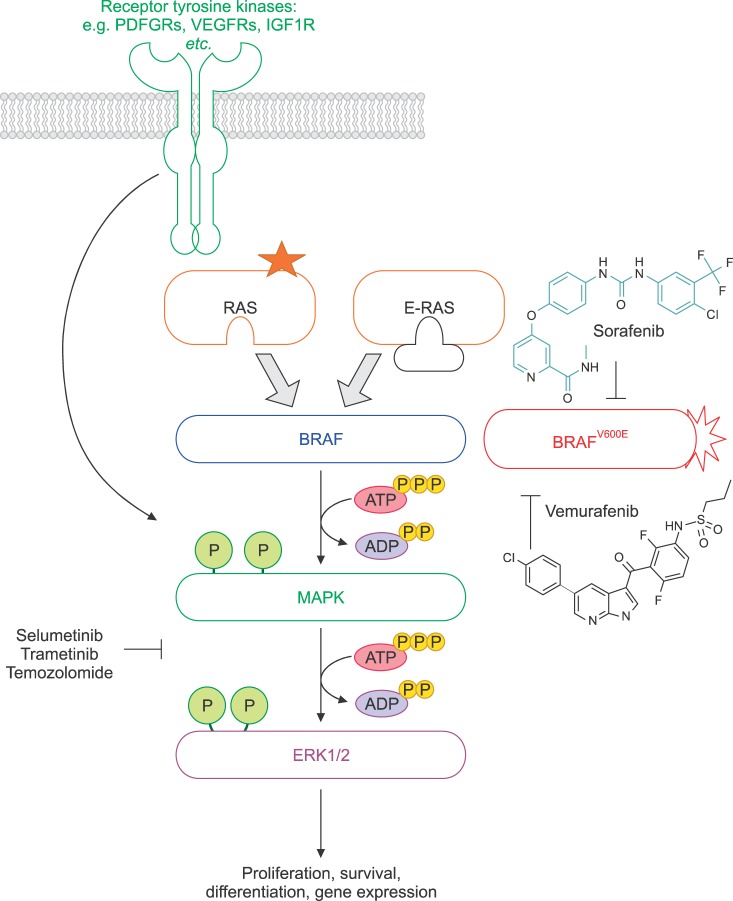
Rapidly accelerated fibrosarcoma (RAF), a proto-oncogene product, and B-RAF are members of the RAF kinase family. This family of RAF proteins A-RAF, B-RAF, and C-RAF is activated by RAS (Rat Sarcoma), which in its GT bound state sets off a cascade of cellular signaling pathways.3 These RAF proteins are growth signal transduction protein kinases and their signaling cascade induces gene expression which alters mutagenesis and may also potentiate oncogenesis.345 BRAF has been known to play a role in regulating the mitogen-activated protein kinases (MAPKs) signaling pathway which affects cell division, differentiation, and secretion.4 MAPK was originally called extracellular signal-regulated kinases (ERKs), and microtubule associated protein kinases (MAPKs), thus the other name of MAPK is MAPK/ERK kinase (MEK).6 Constitutively active B-RAF mutants have been identified in many malignant melanomas which signal cells to grow in excess. The BRAF mutations, followed by MEK cascade activation, play an oncogenic role in malignant melanoma development. Thus, inhibitors of B-RAF were developed as a potential therapy for melanoma. Furthermore, MEK inhibitors are important in this pathway, as MEK is a key downstream signaling mediator of the RAS/RAF pathway.7 Small-molecule inhibitors of MEK have been proposed as anticancer therapeutics, due to the complete abrogation of tumor growth in BRAF-mutant xenografts; whereas, RAS-mutant tumors were only partially inhibited.8 Therefore, MEK inhibitors are considered to be another target for the treatment of BRAF-mutant cancers such as metastatic melanoma.
Go to : 
Vemurafenib is a potent inhibitor of the B-RAFV600E mutant that was first synthesized in early 2005, and it was approved by the U.S. Food and Drug Administration (FDA) for treatment of patients with late stage melanoma and metastatic melanoma.9 Vemurafenib inhibits the active “DFG-in” form of the kinase, which is strongly anchored to the ATP binding domain, unlike sorafenib, which inhibits the inactive form of the kinase domain.5 Selectivity of vemurafenib for B-RAFV600E, over wild-type BRAF, in the biochemical assays is modest; however, its selectivity for B-RAFV600E in melanoma cell lines is remarkable. These drugs potently inhibit MEK phosphorylation, induce cell cycle growth arrest and apoptosis in vitro, and cause tumor regression in vivo, exclusively in B-RAFV600E-positive tumor models.10 Comparatively, vemurafenib only blocks MEK phosphorylation in BRAF mutant cells; while MEK inhibitors block MEK phosphorylation regardless of cellular genotype.10 Since vemurafenib is specifically effective in cells with the BRAF mutation at codon 600, a valine(V) to glutamic acid (E), the proliferation of B-RAFV600E melanoma,101112 colorectal cancer,1314 and papillary thyroid cancer cell lines1516 is inhibited by vemurafenib.
Although vemurafenib is associated with a relatively significant risk reduction compared with sorafenib and/or dacarbazine,17 a resistance to BRAF inhibition in melanoma patients has already been reported.1819 The resistance to BRAF inhibition was mediated through either receptor tyrosine kinase (RTK)-mediated activation of an alternative survival pathway or activated RAS-mediated reactivation of the MEK pathway in melanoma.20 As the MEK pathway is frequently re ignited in relapsed tumors, treatment with vemurafenib combined with BRAF/MEK inhibitors potentially may be warranted, and such studies are underway.2122 Moreover, vemurafenib could be used together with PI3K, AKT, or the mammalian target of rapamycin (mTOR) inhibitors, owing to the co-dependence of melanoma. The cytotoxic T lymphocyte antigen 4 (CTLA4) antibody ipilimumab, could also be used as a target agent when combined with vemurafenib for treatment of metastatic melanoma.23 Ipilimumab was the first check point inhibitor that reached the clinic, gaining FDA and EMA approval for metastatic melanoma in 2011, in addition to being approved by the FDA as an adjuvant for patients with high risk, stage III melanoma.24 This suggests that vemurafenib may be closely associated with the T cell response that is critical for the success of many immunotherapies.25
After gleaning more knowledge of vemurafenib in additional clinical trials, the development of next generation of BRAF inhibitors may be identified as a strategy for improving treatment options for melanoma patients. Accordingly, we believe that the development of vemurafenib may provide a more personalized treatment for metastatic melanoma patients. In the future, drugs that selectively and effectively inhibit functional oncogene products such as B-RAFV600E may be introduced. Future studies will result in a medical environment in which cancers are first analyzed by their genetic driving events, followed by the development of a personalized therapy cocktail to improve the clinical outcome for individual patients.
Go to : 
Dabrafenib (GSK2118436) is another potent and selective B-RAF inhibitor with clinical activity against BRAFV600E melanoma, non-B-RAFV600E-mutated melanoma, and cancers of other organ systems with the V600K mutation, including papillary thyroid, colorectal, and ovarian cancers.26272829 In 2013, the FDA approved dabrafenib as a single agent for the treatment of unresectable or metastatic BRAFV600E melanoma. It is a potent ATP-competitive inhibitor of BRAF kinase, which is highly selective for mutant BRAF in kinase panel screening, cell lines, and xenografts. It is considered to be the first drug of its class to demonstrate activity against metastatic melanoma in the brain. Dabrafenib was synthesized specifically to prevent penetration of the blood-brain barrier due to the potentially neurotoxic effects on abundant wild-type BRAF in normal brain tissue.26
Go to : 
Sorafenib (BAY 43-9006, Nexavar) is a V600E mutant BRAF and CRAF tyrosine kinase inhibitor with specificity for the ATP-binding pocket of RAF.2730 It also targets the vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR).2730 Sorafenib has been shown to disable the B-RAF kinase domain by locking the enzyme in its inactive form and inhibiting the MEK pathway in vitro and in vivo. This is followed by abrogating the downstream MEK pathway and exerting anti-proliferative effects in tumor cell lines and in vivo tumor models.3132 Although sorafenib is approved by the FDA for the treatment of primary liver and kidney cancer,33 it has not shown significant anti-tumor activity in advanced melanoma patients as a single agent. Since its effect on B-RAF is rather weak, its clinical efficacy is generally attributed to targeting non-B-RAF kinases. However, combinational treatment with sorafenib and the alkylating agents dacarbazine or temozolomide resulted in a significant improvement in patients with advanced melanoma.3435 Additionally, several studies have been conducted to confirm the efficacy of sorafenib combined with new drugs for the treatment of melanoma. For example, treatments with sorafenib combined with temsirolimus, riluzole, tipifarnib, and bortezomib have been conducted for advanced melanoma.363738
Go to : 
Monotherapy of advanced melanoma patients with the MEK1/2 inhibitor, selumetinib, has shown weak clinical results.39 There is no significant difference in progression-free survival between patients with unresectable stage III/IV melanoma unselected for BRAF/NRAS mutations, who received therapy with either selumetinib or temozolomide. Some clinical activity of another MEK inhibitor, trametinib, has been observed in K601E and L597Q BRAF mutation-positive metastatic melanoma.40 However, since the PI3K/AKT pathway has been proposed as a survival and escape mechanism for BRAF-mutant melanomas treated with selumetinib, tumor regression appears to correlate with a low phosphorylated AKT presence.41 For this reason, patients with BRAF-mutant melanoma, whose tumors express high levels of phosphorylated AKT, are being treated with a combination of targeting agents against MEK and PI3K/AKT. It was also reported that a combined treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib in patients with BRAFV600E or BRAFV600K positive melanoma showed a 76% of the rate of complete or partial response with combination therapy. This is compared to 54% with dabrafenib monotherapy.542
Go to : 
In order to provide or develop new melanoma therapies using new targeting agents, we need a better understanding of the systemic mechanisms of melanoma oncogenesis, proliferation, and metastases. Although there have been meaningful advances in the knowledge of therapeutic treatment of melanoma patients by several BRAF-mutant specific kinase inhibitors, such as vemurafenib and dabrafenib, acquired resistance of BRAF-mutant melanoma quickly and almost invariably develops. There are numerous potential mechanisms of this tumorigenesis reported, including reactivation of the RAS/RAF/MEK, PI3K/AKT/mTOR pathways, and their downstream signaling targets.304344 One of the best examples is vemurafenib, which has significantly improved survival rates for advanced melanoma patients, though resistance to vemurafenib has been currently reported from several groups.454647
In summary, the signaling through the Ras-Raf-MEK pathways with the somatic BRAFV600E mutation leads to constitutive activity of the kinases. BRAF inhibitors, such as vemurafenib and dabrafenib, are specifically active against BRAF-mutant melanoma; however, in some instances combined treatment with inhibitors of MEK, PI3K/AKT, and/or mTOR may be required due to multiple escape mechanisms for melanoma resistance (Fig. 1). Consequently, targeting the resistance mechanism of BRAF inhibitors through drug treatment combinations could enhance clinical efficacy.
Go to : 
References
1. Ahn A, Chatterjee A, Eccles MR. The slow cycling phenotype: a growing problem for treatment resistance in melanoma. Mol Cancer Ther. 2017; 16:1002–1009. PMID: 28576947.
2. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954. PMID: 12068308.
3. Platz A, Egyhazi S, Ringborg U, Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008; 1:395–405. PMID: 19383313.
4. Wellbrock C, Arozarena I. The complexity of the ERK/MAP-Kinase pathway and the treatment of melanoma skin cancer. Front Cell Dev Biol. 2016; 4:33. PMID: 27200346.
5. Fiskus W, Mitsiades N. B-Raf Inhibition in the Clinic: Present and Future. Annu Rev Med. 2016; 67:29–43. PMID: 26768236.
6. Avruch J, Khokhlatchev A, Kyriakis JM, Luo Z, Tzivion G, Vavvas D, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res. 2001; 56:127–155. PMID: 11237210.
7. An S, Yang Y, Ward R, Liu Y, Guo XX, Xu TR. A-Raf: A new star of the family of raf kinases. Crit Rev Biochem Mol Biol. 2015; 50:520–531. PMID: 26508523.
8. Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006; 439:358–362. PMID: 16273091.
9. Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012; 11:873–886. PMID: 23060265.
10. Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010; 70:5518–5527. PMID: 20551065.
11. Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010; 23:190–200. PMID: 20149136.
12. Søndergaard JN, Nazarian R, Wang Q, Guo D, Hsueh T, Mok S, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med. 2010; 8:39. PMID: 20406486.
13. Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010; 107:14903–14908. PMID: 20668238.
14. Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res. 2010; 16:3329–3334. PMID: 20472680.
15. Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res. 2008; 6:751–759. PMID: 18458053.
16. Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, et al. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab. 2010; 95:450–455. PMID: 19880792.
17. Young K, Minchom A, Larkin J. BRIM-1, -2 and -3 trials: improved survival with vemurafenib in metastatic melanoma patients with a BRAF(V600E) mutation. Future Oncol. 2012; 8:499–507. PMID: 22646765.
18. Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010; 102:1724–1730. PMID: 20531415.
19. Basile KJ, Abel EV, Aplin AE. Adaptive upregulation of FOXD3 and resistance to PLX4032/4720-induced cell death in mutant B-RAF melanoma cells. Oncogene. 2012; 31:2471–2479. PMID: 21996740.
20. Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010; 468:973–977. PMID: 21107323.
21. Kudchadkar R, Paraiso KH, Smalley KS. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 2012; 18:124–131. PMID: 22453012.
22. Ravnan MC, Matalka MS. Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma. Clin Ther. 2012; 34:1474–1486. PMID: 22742884.
23. Trino E, Mantovani C, Badellino S, Ricardi U, Filippi AR. Radiosurgery/stereotactic radiotherapy in combination with immunotherapy and targeted agents for melanoma brain metastases. Expert Rev Anticancer Ther. 2017; 17:347–356. PMID: 28277101.
24. Specenier P. Ipilimumab in melanoma. Expert Rev Anticancer Ther. 2016; 16:811–826. PMID: 27403706.
25. Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010; 70:5213–5219. PMID: 20551059.
26. Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012; 379:1893–1901. PMID: 22608338.
27. Wang H, Quan H, Lou L. AKT is critically involved in the antagonism of BRAF inhibitor sorafenib against dabrafenib in colorectal cancer cells harboring both wild-type and mutant (V600E) BRAF genes. Biochem Biophys Res Commun. 2017; 489:14–20. PMID: 28536078.
28. Williams CB, McMahon C, Ali SM, Abramovitz M, Williams KA, Klein J, et al. A metastatic colon adenocarcinoma harboring BRAF V600E has a durable major response to dabrafenib/trametinib and chemotherapy. Onco Targets Ther. 2015; 8:3561–3564. PMID: 26664139.
29. King AJ, Arnone MR, Bleam MR, Moss KG, Yang J, Fedorowicz KE, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013; 8:e67583. PMID: 23844038.
30. Velho TR. Metastatic melanoma - a review of current and future drugs. Drugs Context. 2012; 2012:212242. PMID: 24432031.
31. Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010; 467:596–599. PMID: 20823850.
32. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809–819. PMID: 20818844.
33. Gang G, Hongkai Y, Xu Z. Sorafenib combined with radiofrequency ablation in the treatment of a patient with renal cell carcinoma plus primary hepatocellular carcinoma. J Cancer Res Ther. 2015; 11:1026.
34. McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008; 26:2178–2185. PMID: 18445842.
35. Romero AI, Chaput N, Poirier-Colame V, Rusakiewicz S, Jacquelot N, Chaba K, et al. Regulation of CD4(+)NKG2D(+) Th1 cells in patients with metastatic melanoma treated with sorafenib: role of IL-15Rα and NKG2D triggering. Cancer Res. 2014; 74:68–80. PMID: 24197135.
36. Lee HJ, Wall BA, Wangari-Talbot J, Shin SS, Rosenberg S, Chan JL, et al. Glutamatergic pathway targeting in melanoma: single-agent and combinatorial therapies. Clin Cancer Res. 2011; 17:7080–7092. PMID: 21844014.
37. Margolin KA, Moon J, Flaherty LE, Lao CD, Akerley WL 3rd, Othus M, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438). Clin Cancer Res. 2012; 18:1129–1137. PMID: 22228638.
38. Kumar SK, Jett J, Marks R, Richardson R, Quevedo F, Moynihan T, et al. Phase 1 study of sorafenib in combination with bortezomib in patients with advanced malignancies. Invest New Drugs. 2013; 31:1201–1206. PMID: 23887852.
39. Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012; 18:555–567. PMID: 22048237.
40. Bowyer SE, Rao AD, Lyle M, Sandhu S, Long GV, McArthur GA, et al. Activity of trametinib in K601E and L597Q BRAF mutation-positive metastatic melanoma. Melanoma Res. 2014; 24:504–508. PMID: 24933606.
41. Catalanotti F, Solit DB, Pulitzer MP, Berger MF, Scott SN, Iyriboz T, et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013; 19:2257–2264. PMID: 23444215.
42. Johnson DB, Flaherty KT, Weber JS, Infante JR, Kim KB, Kefford RF, et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014; 32:3697–3704. PMID: 25287827.
43. Kunz M. Oncogenes in melanoma: an update. Eur J Cell Biol. 2014; 93:1–10. PMID: 24468268.
44. Nikolaou VA, Stratigos AJ, Flaherty KT, Tsao H. Melanoma: new insights and new therapies. J Invest Dermatol. 2012; 132:854–863. PMID: 22217739.
45. Kulkarni A, Al-Hraishawi H, Simhadri S, Hirshfield KM, Chen S, Pine S, et al. BRAF fusion as a novel mechanism of acquired resistance to vemurafenib in BRAFV600E mutant melanoma. Clin Cancer Res. 2017; DOI: 10.1158/1078-0432.CCR-16-0758. [Epub ahead of print].
46. Theodosakis N, Micevic G, Langdon CG, Ventura A, Means R, Stern DF, et al. p90RSK blockade inhibits dual BRAF and MEK inhibitor-resistant melanoma by targeting protein synthesis. J Invest Dermatol. 2017; DOI: 10.1016/j.jid.2016.12.033. [Epub ahead of print].
47. Wu SH, Zhang LN, Speakman JR, Wang DH. Limits to sustained energy intake. XI. A test of the heat dissipation limitation hypothesis in lactating Brandt's voles (Lasiopodomys brandtii). J Exp Biol. 2009; 212:3455–3465. PMID: 19837887.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download