Abstract
The purpose of the present study was to evaluate the correlations between high platelet reactivity (HPR) and the extent of coronary atherosclerosis and periprocedural myonecrosis in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). A total of 485 patients who underwent PCI for ACS was studied. HPR was defined as ≥230 platelet reactivity units (PRU) in point-of-care P2Y12 tested by the VerifyNow assay. The incidence of multi-vessel disease (MVD) was higher in patients with HPR than those with no HPR (56.2% vs 45.8%, p=0.023). PRU values progressively increased with the number of diseased coronary arteries (1-vessel disease 221.8±86.7; 2-vessel disease 239.3±90.1; 3-vessel disease 243.4±84.5; p=0.038 by ANOVA). Multivariate analysis revealed that HPR was independently associated with MVD (Odds ratio 1.48, 95% confidence interval 1.01-2.25, p=0.048). Patients with periprocedural myonecrosis showed significantly higher PRU values compared with those without myonecrosis (258.6±94.5 vs. 228.5±85.6, p=0.013). Multivariate analysis revealed that HPR was an independent predictor for periprocedural myonecrosis as defined as any creatine kinase-myocardial band isoenzyme elevation or troponin T elevation. In conclusion, HPR is associated with MVD and periprocedural myonecrosis in patients with ACS and PCI. Thus, platelet reactivity after treatment with clopidogrel might be associated not only with blood clot formation but also with increased coronary atherosclerotic burden.
Platelet P2Y12 receptor inhibitor non-responsiveness, characterized as high on-treatment platelet reactivity (HPR), has been shown to be correlated with adverse cardiovascular events after acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI).1234 Platelet activation and aggregation in response to endothelial injury, such as plaque rupture or stenting, is responsible for intracoronary blood clot formation, leading to ischemic heart disease. Moreover, growing evidence suggests that platelets are also important mediators of inflammation and play a central role in atherogenesis itself.56 Therefore, HPR is not only associated with myocardial infarction (MI) or stent thrombosis, but may also be associated with increased coronary atherosclerotic burden. However, the potential relationship between residual platelet reactivity after clopidogrel treatment and the extent and severity of coronary atherosclerosis has not been widely investigated.
Therefore, we conducted an observational study involving consecutive ACS patients who underwent PCI. The purpose of the present study was to evaluate the correlation between HPR and the extent and severity of coronary atherosclerosis. In addition, we evaluated whether HPR after clopidogrel might account for an unfavorable periprocedural outcome in patients with ACS who underwent PCI.
We analyzed a single center, consecutive non-ST segment elevation ACS and PCI cohort from December 2012 to August 2014. During the study period, 600 consecutive patients were recruited and followed-up during their clinical course to document patient characteristics, acute therapy, PCI data, and hospital outcomes. Exclusion criteria were patients with ST segment elevation MI, the use of other P2Y12 inhibitors instead of clopidogrel, chronic clopidogrel therapy, use of glycoprotein IIb/IIIa inhibitors, previous coronary artery bypass surgery, concomitant proton pump inhibitor use, or lack of laboratory data including platelet reactivity unit (PRU). As a result, 485 patients were analyzed. All patients provided informed consent for the processing of their anonymous data, according to a protocol approved by the Institutional Review Boards of Wonkwang University Hospital.
In all patients, aspirin (300 mg/day) and clopidogrel (300 mg/day) were loaded before the procedure. An intravenous bolus of 5,000 U of unfractionated heparin was given, and then additional heparin boluses were given to maintain activated clotting time >300 s during the procedure. Coronary angiography and stent implantation were performed using standard interventional techniques. Aspirin (100 mg/day), clopidogrel (75 mg/day) and statins were prescribed to all patients after the procedure.
The occurrence of angiographic complications during PCI, including side branch occlusion, slow or no reflow, major dissection, and distal embolization were recorded. Creatine kinase-myocardial band isoenzyme (CK-MB) and troponin T were measured before, at 6 hours, 24 hours and 48 hours after PCI. Additional samples were obtained if the patients showed signs or symptoms of myocardial ischemia.
Platelet reactivity after successful PCI was assessed by VerifyNow P2Y12 assays (Accumetrics, San Diego, CA, USA). Blood samples were taken to measure platelet reactivity at 48 h after 300 mg clopidogrel loading. A washout period was required if platelet glycoprotein IIb/IIIa inhibitors were used, and thus no patients receiving glycoprotein IIb/IIIa inhibitor were enrolled. VerifyNow P2Y12 baseline reactivity, PRU, and P2Y12 percent inhibition were measured to assess platelet function. In this study, platelet reactivity ≥230 PRU was defined as HPR.7
The primary end point of the study was the correlation between the extent of atherosclerotic coronary artery disease (defined as coronary artery stenosis >50% in major epicardial vessel and branch) and HPR.
The secondary end point was the occurrence of periprocedural myonecrosis, defined as any increase of cardiac biomarkers above the upper limit of normal or ≥20% increase of elevated baseline value.8 Other secondary end points included periprocedural MI, defined as a postprocedural increase of CK-MB over 3 times higher the normal upper limit in patients with normal baseline enzyme levels. In patients with elevated baseline levels of CK-MB, MI was defined as a subsequent increase that was more than 3 times greater than the baseline CK-MB value and an additional increase in a second sample.
All measurements were represented as mean±standard deviation or absolute number (percentage). Inter-group analysis was performed using independent t-test and χ2 test, which were conducted using SPSS 19.0 for Window (SPSS Inc., Chicago, IL). The hypothesis of the study was that patients with multi-vessel disease (MVD) would present a 15% greater incidence in the HPR group as compared with patients with single-vessel disease (SVD) after clopidogrel loading. Assuming a 50% incidence of MVD, we calculated that at least 153 patients should be included in each group to provide 80% power to detect between group differences, with a two-sided alpha of 0.05.9 All clinical and procedural variables that showed a significant univariate association with MVD and periprocedural myonecrosis (p<0.1) were entered in a multivariable logistic regression model. For continuous variables, the first or third tertile was used as the cut-off point in logistic regression analysis. Statistical significance was set at p<0.05.
The mean PRU value in the overall population was 232.1±87.1, and 260 patients (53.6%) showed HPR (PRU≥230). About 25% of patients presented as MI (non-ST segment elevation MI). Patient characteristics, according to HPR, are shown in Table 1. HPR was observed more commonly in elderly patients, female patients, current smokers, and in patients with previous ischemic stroke. HPR was associated with low hemoglobin and high brain natriuretic peptide levels.
The incidence of MVD was higher in patients with HPR than those with no HPR (56.2% vs 45.8%, p=0.023) (Table 2). Patients with MVD showed significantly higher PRU values compared with those with SVD (241.9±86.6 vs. 221.8±86.7, p=0.011; Fig. 1). The PRU values progressively increased with number of diseased coronary arteries (1-vessel disease 221.8±86.7; 2-vessel disease 239.3±90.1; 3-vessel disease 243.4±84.5; p=0.038 by ANOVA). Multivariate analysis revealed that HPR was independently associated with MVD (Odds ratio 1.50, 95% confidence interval 1.01-2.24, p=0.047; Table 3).
Periprocedural myonecrosis occurred in 58 patients (12.0%) based on any CK-MB elevation and in 172 patients (35.5%) based on any troponin T elevation. Patients with periprocedural myonecrosis showed significantly higher PRU values compared with those without myonecrosis (258.6±94.5 vs. 228.5±85.6, p=0.013). Regarding CK-MB elevation, the incidences of any CKBM elevation (15.0% vs. 8.4%, p=0.026), 2 times the elevation of CK-MB (5.4% vs. 2.2%, p=0.073), and 3 times the elevation of CK-MB (2.3% vs. 0.4%, p=0.129) were higher in patients with HPR compared with those with no HPR (Fig. 2). Regarding troponin T elevation, incidences of any troponin T elevation (40.8% vs. 29.3%, p=0.009), 3 times the elevation of troponin T (14.2% vs. 7.1%, p=0.012), and 5 times the elevation of troponin T (10.8% vs. 5.3%, p=0.030) were significantly higher in patients with HPR compared with those with no HPR. Multivariate analysis revealed that HPR was an independent predictor for periprocedural myonecrosis as defined as any CK-MB elevation or troponin T elevation (Table 4).
This study demonstrated that HPR was associated with MVD and periprocedural myonecrosis in patients with ACS and PCI. Thus, platelet reactivity after clopidogrel treatment was associated not only with blood clot formation but also with increased coronary atherosclerotic burden.
Blood platelets actively participate in vascular atherosclerosis. In vitro studies demonstrated that interactions between activated platelets, leukocytes and endothelial cells triggered autocrine and paracrine signals. As a resul, leukocyte recruitment occurred at and into the vascular wall.1011 Moreover, with chemical signaling, direct physical interaction may also support to atherosclerosis, through platelet adhesion molecules and platelet granule release.12 These processes may contribute to foam cell formation and accelerated atherogenesis. Afek et al. reported that antiplatelet therapy could decrease plaque size and improved plaque stability in animal models of accelerated atherogenesis.6 Therefore, increased platelet reactivity may potentiate arterial thrombosis, inflammation, and atherosclerotic progression.
HPR has been defined as a high level of platelet reactivity that is measured during steady state platelet inhibition after receiving a loading dose of an antiplatelet agent.13 Several studies have reported associations between HPR and increased atherosclerotic burden. Keating et al.14 demonstrated that platelet reactivity progressively increases with the number of vascular beds affected by atherosclerosis (cerebral, cardiac, peripheral). Mangiacapra et al.9 reported that MVD was associated with an increased rate of HPR in patients with stable angina. Yun et al. demonstrated that HPR was associated with high plaque burdens and high incidences of fibroatheroma in virtual-histology intravascular study.16 Our findings confirmed these observations and extended them to ACS patients.
Several mechanisms have been described for the suboptimal response to clopidogrel, including genetic, cellular, and clinical factors.17 Increased platelet reactivity was commonly observed in specific clinical scenarios such as ACS, increased body mass index, heart failure, chronic kidney disease and diabetes.1315 In our study conducted in ACS patients, patients with HPR had a higher incidence of hypertension, smoking, previous ischemic stroke, and anemia compared to that in patients without HPR. These results may be caused by different patients' populations, and these different incidences of risk factors according to PRU might have contributed to the higher incidence of MVD in the HPR group.
In our study, HPR was significantly associated with periprocedural myonecrosis, even with different degrees of post-procedural cardiac biomarker elevations. Patti et al.18 reported that HPR (PRU≥240) was associated with a higher incidence of 30-day major adverse cardiac events after PCI; this outcome was mainly driven by an increased risk of periprocedural MI. In view of the more extensive and complex coronary manipulation, patients with MVD might be expected to have a higher risk of periprocedural myonecrosis. Additionally, higher PRU values were observed in MVD patients, and it suggests that suboptimal platelet response to clopidogrel in this clinical setting might be responsible for the higher risk of periprocedural MI. In fact, our study confirmed the results of previous studies which demonstrated that HPR was a strong predictor of periprocedural myonecrosis.91920
Our study has several limitations. This was single center, retrospective study. Clinical follow-ups of the patients were not performed, and the long-term clinical significance of HPR was not evaluated. However, the relation between platelet reactivity and the occurrence of major adverse cardiac events was already reported, regardless of a specific PRU cut-off.72122 Moreover, periprocedural myocardial injury also has been reported as risk factor of major adverse events.23 Our study suggested only correlative associations; the cause-and-effect relationship between platelet reactivity and coronary artery disease burden remains theoretical.
In conclusion, HPR was shown to be associated with MVD and periprocedural myonecrosis in patients with ACS and PCI. It demonstrated the need for more potent antiplatelet strategies in this clinical setting. Further studies are required to define the causal mechanisms between platelet reactivity and the development and progression of atherosclerosis.
Figures and Tables
FIG. 1
Associations between platelet reactivity units (PRUs) and coronary artery disease. SVD: single-vessel disease, MVD: multivessel disease, 1VD: 1-vessel disease, 2VD, 2-vessel disease, 3VD: 3-vessel disease.
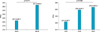
FIG. 2
Incidence of periprocedural myonecrosis according to platelet reactivity. HPR, high platelet reactivity, CK-MB: creatine kinase myocardial band isoenzyme.

TABLE 1
Baseline characteristics

MI: myocardial infarction, PCI: percutaneous coronary intervention, WBC: white blood cell, hsCRP: high-sensitivity C-reactive protein, LDL: low density lipoprotein, BNP: brain natriuretic peptide, CK-MB: creatine kinase myocardial band isoenzyme, ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker.
References
1. Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. 2005; 3:85–92.

2. Gurbel PA, Bliden KP, Samara W, Yoho JA, Hayes K, Fissha MZ, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol. 2005; 46:1827–1832.

3. Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004; 109:3171–3175.

4. Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007; 49:657–666.

5. Gawaz M, Stellos K, Langer HF. Platelets modulate atherogenesis and progression of atherosclerotic plaques via interaction with progenitor and dendritic cells. J Thromb Haemost. 2008; 6:235–242.

6. Afek A, Kogan E, Maysel-Auslender S, Mor A, Regev E, Rubinstein A, et al. Clopidogrel attenuates atheroma formation and induces a stable plaque phenotype in apolipoprotein E knockout mice. Microvasc Res. 2009; 77:364–369.

7. Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011; 58:1945–1954.

8. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Joint ESC/ACCF/AHA/WHF Task Force for Universal
Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012; 60:1581–1598.

9. Mangiacapra F, De Bruyne B, Muller O, Trana C, Ntalianis A, Bartunek J, et al. High residual platelet reactivity after clopidogrel: extent of coronary atherosclerosis and periprocedural myocardial infarction in patients with stable angina undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2010; 3:35–40.
10. Seizer P, Gawaz M, May AE. Platelet-monocyte interactions--a dangerous liaison linking thrombosis, inflammation and atherosclerosis. Curr Med Chem. 2008; 15:1976–1980.

11. Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000; 95:1158–1166.

12. Linden MD, Jackson DE. Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol. 2010; 42:1762–1766.

13. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007; 49:1505–1516.
14. Keating FK, Whitaker DA, Kabbani SS, Ricci MA, Sobel BE, Schneider DJ. Relation of augmented platelet reactivity to the magnitude of distribution of atherosclerosis. Am J Cardiol. 2004; 94:725–728.

15. Park KW, Park JJ, Jeon KH, Kang SH, Oh IY, Yang HM, et al. Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc Ther. 2012; 30:5–11.

16. Yun KH, Mintz GS, Witzenbichler B, Inaba S, Shimizu T, Metzger DC, et al. Relationship between platelet reactivity and culprit lesion morphology: an assessment from the ADAPT-DES intravascular ultrasound substudy. JACC Cardiovasc Imaging. 2016; 9:849–854.
17. Price MJ, Berger PB, Angiolillo DJ, Teirstein PS, Tanguay JF, Kandzari DE, et al. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: design and rationale of the GRAVITAS trial. Am Heart J. 2009; 157:818–824. 824.e1

18. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008; 52:1128–1133.

19. Cuisset T, Hamilos M, Sarma J, Sarno G, Wyffels E, Vanderheyden M, et al. Relation of low response to clopidogrel assessed with point-of-care assay to periprocedural myonecrosis in patients undergoing elective coronary stenting for stable angina pectoris. Am J Cardiol. 2008; 101:1700–1703.

20. Mangiacapra F, Barbato E, Patti G, Gatto L, Vizzi V, Ricottini E, et al. Point-of-care assessment of platelet reactivity after clopidogrel to predict myonecrosis in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2010; 3:318–323.

21. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013; 382:614–623.

22. Lee K, Lee SW, Lee JW, Kim SY, Youn YJ, Ahn MS, et al. The significance of clopidogrel low-responsiveness on stent thrombosis and cardiac death assessed by the verifynow P2Y12 assay in patients with acute coronary syndrome within 6 months after drug-eluting stent implantation. Korean Circ J. 2009; 39:512–518.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download