Abstract
Invasive lobular carcinoma (ILC) is the second most common kind of breast cancer. Diffusion weighted imaging (DWI) and positron emission tomography/computed tomography (PET/CT) are functional modalities for presenting the biological characteristics of breast cancer. The purpose of this article is to study the relationship between DWI or PET/CT and ILC's prognostic factors. The relationship between the apparent diffusion coefficient (ADC) values, standard uptake value (SUV)max and prognostic factors of ILC were statistically evaluated. The ADC values were lower in mass types of ILC. SUVmax was statistically higher in grade 3 and 4 background parenchymal enhancement and positive lymph node metastasis. ADC values of DWI and SUVmax of PET/CT can be helpful in the prediction of the prognosis of ILC.
Breast cancer tumors are very heterogeneous, and show variable behavior, progression, and response to therapy. Prediction of these variables is very important in deciding therapy. Conventional prognostic factors of breast cancer are lymph node status, primary tumor size, and primary tumor grade. Immunohistochemical prognostic factors of breast cancer are the estrogen receptor (ER), the progesterone receptor (PR), the human epidermal growth factor receptor (HER) 2, Ki-67, and the epidermal growth factor receptor (EGFR).
Diffusion weighted imaging (DWI) and 18F-fluorodeoxygluxose positron emission tomography/computed tomography (18F-FDG PET/CT) are functional modalities presenting biological characteristics of breast cancer. DWI is a modality used to evaluate the micro-structural characteristics of water diffusion in biological tissues.1 The apparent diffusion coefficient (ADC) is calculated using DWI. Malignant tumors with highly cellular lesions display low ADC values due to their inverse relationship with tumor cellularity.23 18F-FDG PET/CT reflects the increased glucose levels in cancer cells and is used in the diagnosis of lesions, staging, recurrence, and treatment response.4 FDG uptake aids in predicting the prognosis for primary breast cancer and is associated with several histopathological and immunohistochemical prognostic factors.56 The standardized uptake value (SUV) is used in PET imaging for simple semiquantitative analysis. ADC and SUV values are important in predicting the prognosis in breast cancer. Many studies have reported the relationship between ADC values or SUV and immunohistochemical prognostic factors in breast cancer.23456
Invasive lobular carcinoma (ILC) is the second most common type of breast cancer, making up 5-15% of all breast cancer diagnoses.78 ILC has a different morphology compared to invasive ductal carcinoma (IDC), and is characterized by small, round cells that invade the stroma singly in a “single-file” pattern resulting in linear stands.78 Those cells don't destroy the anatomical structure, but infiltrate through stroma and adipose tissue without desmoplastic reaction, which does not result in the formation of a mass.910 Therefore, both the clinical and radiological detection of ILC is difficult.910 The distribution of ILC tends to be multifocal and multicentric with a unique metastatic spread pattern,11 and the potential for it being in both breasts is high.111213 The prognosis for ILC is known to be similar or better to that of IDC.121314 Biologically, ILC tumors are usually of a low histological grade, they tend to have less lymph node metastasis, and tend to be ER positive, PR and HER2 negative, as well as have overexpression of p53, EGFR.141516
To our knowledge, research about predicting the prognosis of breast cancer with DWI and FDG PET/CT is mainly focused on IDC. Since ILC is the second most common breast cancer and has very unique characteristics, we studied the relationship between DWI or PET/CT and ILC's prognostic factors.
This was a retrospective study, in which information was obtained from the pathology databases of 4 hospitals. Histology reports were collected from patients with ILC who had MRIs including DWI and FDG PET/CT and had their diagnoses confirmed by biopsy and underwent surgery between June 2007 and September 2013. A total of 86 patients were enrolled in the study. Among them, 34 patients were available for ADC value measurement. The others did not have DWI, or the ADC value could not be measured on them because the machine which incapable of measuring the ROI (n=52). The SUVmax was measured in all 86 patients. Both ADC value and SUVmax were measured in 30 patients.
The MRIs were taken using a 1.5 T scanner (Achieva; Philips Medical system, Best, the Netherlands) and a 3.0 T scanner (Magnetom Verio; Siemens Medical Solutions, Erlangen, Germany) equipped with a breast coil. The MRIs with the Achieva scanner were performed using the following sequences: sagittal, fat-suppressed, fast spin-echo T2-weighted imaging sequence with a TR/TE of 6000/100, flip angle of 90, 30 slices with an FOV of 320 mm, matrix size of 424×296, 1 NEX, slice thickness of 4 mm with 0.1 mm interslice gap, acquisition time of 2 minutes 56 seconds; axial DWI with a single-shot echo planar imaging (EPI) with b of 0 and 1,000 second/mm2, TR/TE of 8269.9/70.4, FOV of 363 mm, matrix size of 128×130, 3 NEX, slice thickness of 4 mm with a 1-mm slice gap, and an acquisition time of 4 minutes 33 seconds; pre- and dynamic axial T1-weighted three-dimensional, fat-suppressed, fat-spoiled gradient-echo sequence with TR/TE of 6.9/3.4, flip angle of 12°, slice thickness of 2.0 mm, acquisition time of 1 minute 31 seconds, obtained before and at 0, 91, 182, 273, 364 and 455 sec after a rapid bolus injection of 0.2 mmol/kg body weight of Gadobutrol (Gadovist/Gadovist, Bayer Pharma AG, Berlin, Germany).
The MRIs from the Verio scanner were acquired using the following sequences: axial, turbo spin-echo T2-weighted imaging sequence with a TR/TE of 4530/93, flip angle of 80°, 34 slices, FOV of 320 mm, matrix size of 576×403, 1 NEX, slice thickness of 4 mm, acquisition time of 2 minutes 28 seconds; axial DWI with echo planar imaging (EPI) with b of 0 and 750 seconds/mm2, TR/TE of 9700/87, FOV of 340 mm, matrix size of 192×66, 4 NEX, slice thickness of 4 mm with a 1-mm slice gap, and acquisition time of 2 minutes 45 seconds; pre- and post-contrast, axial T1-weighted flash three-dimensional, VIBE sequence with TR/TE of 4.4/1.7, flip angle of 10°, slice thickness of 1.2 mm, acquisition time of 7 minutes 7 seconds, obtained before and at 7, 67, 127, 187, 247 and 367 sec after contrast injection.
PET/CT Studies were acquired using combined PET/CT in-line systems: either Biograph Duo or Biograph Truepoint (Siemens Medical Solutions, Knoxville, TN). The acquisition time was 2 to 3 minutes per bed position. All patients were in a supine position during the PET/CT scanning. The CT scan began at the orbitomeatal line and progressed to the proximal thigh (130 kVp, 80 mAs, and 5 mm slice thickness; 120 kVp, 50 mAs, and 5 mm slice thickness). The PET scan followed immediately over the same body region. The CT data was used for attenuation correction, and the images were reconstructed using a standard ordered-subset expectation maximization (OSEM) algorithm. The axial spatial resolution was 6.5 mm or 4.5 mm at the center of the field of view, respectively.
DWIs were obtained along each of the x-, y- and z-axes. The ADC value was calculated according to the formula: ADC=[1/(b2−b1)] ln (S2/S1), where S1 and S2 are the signal intensities in the regions of interest (ROI) obtained by two gradient factors, b2 and b1 (b1=0 and b2=1,000 s/mm2 for the 1.5 T scanner; b1=0 and b2=750 s/mm2 for the 3.0 T scanner). The ADC value measurements were available in 3 hospital cases. For the measurement of the ADC value, three breast radiologists manually focused on a region of interest (ROI) that was slightly smaller than the solid portion of the tumor to ensure that cystic, necrotic portions of normal parenchyma were not included.710 The mean ADC values were obtained.
Each background parenchymal enhancement (BPE) was assessed using breast MRI. BPE was defined as normal breast parenchymal enhancement and visually assessed in early post-contrast fat-suppressed T1WI or subtraction images.17 BPE was classified by grade 1, minimal enhancement (≤25% enhancement of glandular tissue), grade 2, mild enhancement (26-50% enhancement of glandular tissue), grade 3, moderate enhancement (51-75% enhancement of glandular tissue) and grade 4, marked enhancement (>75% enhancement of glandular tissue) in references with ACR BI-RADS criteria.1718
Two breast radiologists reviewed the PET/CT report papers and SUVmax of the patients.
Both ADC values and SUVmax were evaluated on the primary breast cancers, not on metastatic lesions.
Pathologic reports were reviewed to determine tumor size, lymph node metastasis, and histological grade. Immunohistochemistry was used to test for the expression of the following molecular markers: ER, PR, HER2, Ki-67, and EGFR. ER and PR positivity were defined as the presence of 10% or more positively stained nuclei in ten high-power fields. The intensity of c-erbB-2 staining was scored as 0, 1+, 2+, or 3+. Tumors with 2+ or 3+ scores were classified as HER2 positive, and tumors with 0 or 1+ were negative. EGFR was considered positive if membrane staining was observed. A Ki-67 of >=15% was considered positive expression.
Data is presented as the median and range for continuous variables and frequency with percentage for categorical variables.
To examine whether the ADC value and SUVmax could provide prognostic information, the differences in ADC value and SUVmax between each prognostic group were analyzed. In cases in which the prognostic groups were classified as the positive group and the negative group, the Mann-Whitney U test and Kruskal-Wallis test for variables with non-normal distribution were used. To evaluate the correlation between ADC value and SUVmax, Spearman's correlation coefficient was used. The statistical analyses above were performed with SAS software, version 9.1 (SAS Institute Inc. Cary, NC). A two-tailed p-value<0.05 was considered statistically significant.
The mean age of the 86 patients was 53 years (range, 35 to 77 years). The mean tumor size was 2.8 cm. ADC mean value and SUVmax were 1,117×10−6 mm2/s and 3.4, respectively.
Histologically, 16.3% (14/86) of tumors were well differentiated, 74.4% (64/86) were moderate, and 9.3% (8/86) were poorly differentiated. In the immunohistochemical study, ER and PR positive tumors were present in 81 (94%) and 67 patients (78%), respectively; HER2 negatives tumors were present in 70 patients (81%); EGFR negativities tumors were present in 81 patients (94%). Positive Ki-67 expression was in 88% (71/86) of tumors.
Statistical analysis found that ADC was lower in mass type (Fig. 1) and higher in non-mass type (p=0.047; Fig. 2). SUVmax showed statistically high values in grade 3 and 4 BPE (Fig. 1) and positive lymph node metastasis (Table 1). Even though there was no statistical significance in relation to tumor type, mass type tended to show higher SUV (3.7) compared to non-mass type (2.5) (p=0.098). SUVmax was higher when Ki-67 was lower than 15%, but there was no statistical significance (p=0.069; Fig. 1, and 2).
In our study, ILC had a statistically higher ADC value in the non-mass type. Imamura et al.19 reported that because of the volume-averaging effects, contamination of the background breast parenchymal tissue in measuring the region of interest may explain the false-negative results using the ADC value criteria. They also found that slice thickness was one factor influencing the spatial resolution and volume averaging effects on an image, which needs thinner slices in non-mass like enhancement patterns. Cheng et al.20 found that partial volume had an effect on non-mass like enhancement lesion, which had fat or normal fibroglandular tissue interposing between the lesions, which increased the ADC measurement. Furthermore, they presented that using different ADC cutoff points for different MRI lesion types would be helpful in predicting malignancy. Woodhams et al.21 said the size of an ILC might be underestimated using DWI because it is less conspicuous than IDC. They said that this inaccuracy might be due to the distribution of infiltrating cells resulting in single lines of low cellularity.
High ADC values of ILC in a non-mass like enhancement pattern may be due to the tendency of ILC cells to infiltrate, resulting in an inaccurate measurement of ADC values in non-mass like enhancement patterns of ILC. Further investigation to determine more specific and accurate methods to measure the true lesion in non-mass like enhancements is needed.
ADC values are negatively correlated to tumor cellularity.2223 Tumor cellularity is an important indicator of histological grade.324 Some reports cited that tumors with a higher histological grade showed a lower ADC value in IDC.422 The histological grade of ILC is usually of a lower nuclear grade and has lower mitotic counts than those of IDC.1415 There are no reports in the literature comparing ADC values between ILC and IDC. Tzias et al.25 cited on their poster exhibition that the ADC mean value for ILC was higher than that of IDC, but they were not significantly different. In our study, most ILC tumors were of a lower histological grade (grade 1 & 2, 91%), and 88% of ILC were Ki-67 negative. We didn't compare ADC values between ILC and IDC, but we studied the correlation between ADC values and histological grade, including histological grade marker ki-67, in ILC. Even though a lower ADC value was associated with a lower histological grade and lower Ki-67 (less than 15%), there was no statistical significance.
ADC is statistically correlated with hormone status in IDC.326 Some reports about the relationship between ADC values and hormone status in IDC cited that a lower ADC value was related to positive ER and negative HER2 statuses.326 The reason why a lower ADC value is observed in tumors with a positive ER status is that the estrogen receptor blocks the angiogenic pathway, decreasing perfusion, and since HER2 induces angiogenesis, a negative HER2 status is related to a lower ADC value.327 The hormone status of ILC is known as ER, PR positive, and HER2 negative in most cases.1516 In our study, ER positive, PR positive, and HER2 negative was present in 94%, 78% and 81% of tumors, respectively, which was similar to the results of previous studies. ADC values of ER positive, PR positive, and HER2 negative cases were lower than other cases (Table 1). However, hormone status in ILC was not correlated with the ADC value.
To accurately study the relationship between histological grades or hormone statuses and ADC values, errors in measuring ADC values, especially in non-mass like enhancement tumor patterns, should be reduced. For this, applications of different cutoff values for non-mass like enhancement patterns or measurements of ADC values using thinner sections with repetitive regions of interest are needed.
SUVmax was associated with lymph node metastasis. Lymph node metastasis is an important prognostic factor, and the more metastatic lymph nodes, the worse the prognosis.22 In IDC, SUVmax is associated with lymph node metastasis.456 Hormone receptor status and histological grade are known to be associated with SUVmax in IDC. However, in our study, the above factors were not correlated with SUVmax. Tumor size also was not associated with SUVmax.
FDG uptake in lobular carcinoma has been found to be lower than that of IDC. And this finding accounts for the higher rate of false-negative results of ILC.28 Kumar et al.29 said that lower SUVs in lobular cancers might be explained by lower tumor cell densities and diffuse tissue infiltration. Avril et al.28 explained that non-nodular tumors are more influenced by partial-volume effects, and SUV measurements are greatly affected by partial-volume effects. Thus, in our study, the lower SUV of ILC might be one of the reasons for there being less relevance between SUVmax and prognostic factors of ILC.
However, the lower FDG uptake and higher false-negativities of ILC cannot be a reason for giving up PET studies. Diagnosis and evaluation of ILC is also difficult on the other images such as mammography, sonography, and MRI. Even though FDG uptake of ILC is lower than IDC, PET was suggested to improve diagnostic procedures of malignant tumors, detection regional lymph node metastases and whole body staging. Also, for breast cancers, a combination of PET with MRI can provide the best of the two modalities, resulting in high specificity and high sensitivity, as compared with PET or MRI alone.
SUVmax was correlated with BPE in ILC. BPE is amount of fibroglandular tissue seen on MRIs after contrast enhancement. It is classified as minimal, mild, moderate, or marked enhancement according to the degree of normal breast parenchymal enhancement.1718 Recent papers reported the relationship between BPE and breast cancer risk.17 Cho et al.30 investigated BPE and prognostic factors, and found that BPE is related to the occurrence of breast cancer, but found no relationship between BPE and prognostic factors. They reported that BPE might be related with vascular volume/flow factors.30 Until now, the relationship between BPE and SUV has not been studied. Since there have been no reports about SUV and BPE, we hope our results can help develop further studies looking at the relationship between BPE and prognostic factors.
Among the 86 patients, only 30 patients took both PET and MRI examinations. And the number of patients who took PET scans was different to those who took MRIs.
In the non-mass like enhancement pattern of ILC, precise ROI measurement of lesion was difficult. As mentioned above, more technical methods to measure smaller lesions are needed.
In conclusion, ADC values from DWI and SUVmax of FDG PET/CT correlated with some prognostic factors of ILC. DWI and FDG PET/CT may play a complementary role in predicting the prognosis of ILC.
Figures and Tables
FIG. 1
A 64-year-old woman with invasive lobular carcinoma of the left breast. (A) ADC map shows RIO for measuring the mean ADC value. The calculated ADC value was 1,115×10−6 mm2/s, which was slightly higher than mean ADC value (1,117×10−6 mm2/s). (B) Axial enhanced T1-weighted image after two minutes of contrast injection demonstrates an irregular heterogeneous enhancing mass in the left breast. Background parenchymal enhancement was grade 3, moderate enhancement. (C) FDG PET/CT image shows FDG uptake in the left breast with measured SUVmax as 3.7. On histologic examination, axillary LN metastasis was noted in two LNs. The results of immunohistochemical study were ER (+), PR (+), HER2 (−), EGFR (+) and Ki-67 2%. ADC: apparent diffusion coefficient, EGFR: epidermal growth factor receptor, ER: estrogen receptor, FDG PET/CT: 18 F-fluorodeoxygluxose positron emission tomography/computed tomography, HER2: human epidermal growth factor receptor 2, EGFR: epidermal growth factor receptor, LN: lymph node, PR: progesterone receptor, SUVmax: maximum standardized uptake value.
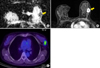
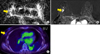
FIG. 2
A 71-year-old woman with invasive lobular carcinoma of the right breast. (A) On ADC map, calculated ADC value by ROI was 1,260×10−6 mm2/s, which was higher than mean ADC value (1,117×10−6 mm2/s). (B) Axial enhanced T1-weighted image shows nonmass like enhancement in the right breast. BPE was grade 1, minimal enhancement. (C) FDG PET/CT image shows minimal FDG uptake in the right breast with measured SUVmas as 1.3. On histologic examination, there was no axillary LN metastasis. Immunohistochemical study showed ER (+), PR (−), HER2 (−), EGFR (+) and Ki-67 10%. ADC: apparent diffusion coefficient, EGFR: epidermal growth factor receptor, ER: estrogen receptor, FDG PET/CT: 18 F-fluorodeoxygluxose positron emission tomography/computed tomography, HER2: human epidermal growth factor receptor 2, EGFR: epidermal growth factor receptor, LN: lymph node, PR: progesterone receptor, SUVmax: maximum standardized uptake value.
References
1. Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging. 2009; 30:615–620.

2. Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009; 36:200–208.

3. Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011; 33:102–109.

4. Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010; 37:2011–2020.

5. Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998; 82:2227–2234.

6. Ikenaga N, Otomo N, Toyofuku A, Ueda Y, Toyoda K, Hayashi T, et al. Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg. 2007; 73:1151–1157.

7. Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979; 3:467–488.

8. Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975; 36:1–85.

9. Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993; 161:957–960.

10. Yeatman TJ, Cantor AB, Smith TJ, Smith SK, Reintgen DS, Miller MS, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg. 1995; 222:549–559. discussion 559-61.
11. du Toit RS, Locker AP, Ellis IO, Elston CW, Nicholson RI, Blamey RW. Invasive lobular carcinomas of the breast--the prognosis of histopathological subtypes. Br J Cancer. 1989; 60:605–609.

12. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996; 77:113–120.

13. Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW, Stewart HJ. Infiltrating lobular carcinoma of the breast: an evaluation of the incidence and consequence of bilateral disease. Br J Surg. 1983; 70:513–516.

14. Toikkanen S, Pylkkänen L, Joensuu H. Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Cancer. 1997; 76:1234–1240.

15. Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005; 23:41–48.

16. Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011; 136:88–97.

17. King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011; 260:50–60.

18. Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am. 2007; 45:863–880.

19. Imamura T, Isomoto I, Sueyoshi E, Yano H, Uga T, Abe K, et al. Diagnostic performance of ADC for Non-mass-like breast lesions on MR imaging. Magn Reson Med Sci. 2010; 9:217–225.

20. Cheng L, Bai Y, Zhang J, Liu M, Li X. Difference of apparent diffusion coefficient in breast mass and non-mass like enhancement lesions. Proc Intl Soc Mag Reson Med. 2011; 19:3084.
21. Woodhams R, Ramadan S, Stanwell P, Sakamoto S, Hata H, Ozaki M, et al. Diffusion-weighted imaging of the breast: principles and clinical applications. Radiographics. 2011; 31:1059–1084.

22. Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010; 23:619–623.

23. Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008; 7:23–29.

24. Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the Apparent Diffusion Coefficient (ADC) values for breast tumors. Korean J Radiol. 2007; 8:390–396.

25. Tzias D, O'flynn E, Allen S, Wilson R. Apparent diffusion coefficient values from diffusion weighted imaging for invasive lobular cancer of the breast. ECR. 2013; DOI: 10.1594/ecr2013/C-0987.
26. Choi BB, Kim SH, Kang BJ, Lee JH, Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012; 10:126.

27. Ludovini V, Sidoni A, Pistola L, Bellezza G, De Angelis V, Gori S, et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res Treat. 2003; 81:159–168.

28. Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001; 42:9–16.
29. Kumar R, Lal N, Alavi A. 18F-FDG PET in detecting primary breast cancer. J Nucl Med. 2007; 48:1751. author reply 1752.

30. Cho GY, Moy L, DeGregorio S, Kim S, Moccaldi M, Kwon J, et al. Background Parenchymal Enhancement (BPE) in healthy subjects and breast cancer patients: a quantitative evaluation and comparison with diffusion-weighted imaging. Paper presented at: 20th Scientific Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM). 2012 May 5-11; Melbourne: Australia;p. 0448.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download