Abstract
Malignant melanoma is a rare disease in Asians but potentially the most aggressive form of skin cancer worldwide. It can occur in any melanocyte-containing anatomic site. Four main cutaneous melanoma subtypes are recognized: lentigo maligna melanoma, superficial spreading melanoma, acral lentiginous melanoma (ALM), and nodular melanoma. Generally, excessive exposure to ultraviolet (UV) radiation increases the risk of melanoma. The exception is ALM, which is the most common melanoma subtype in Asians and is not associated with UV radiation. ALM presents as dark brownish to black, irregular maculopatches, nodules, or ulcers on the palms, soles, and nails. The lesions may be misdiagnosed as more benign lesions, such as warts, ulcers, hematomas, foreign bodies, or fungal infections, especially in amelanotic acral melanomas where black pigments are absent. The aim of this brief review is to improve understanding and the rate of early detection thereby reducing mortality, especially regarding cutaneous melanoma in Asians.
Melanoma develops from melanocytes and is potentially the most aggressive form of skin cancer worldwide. It can occur in any melanocyte-containing anatomic site, such as the basal cell layer of the epidermis, hair follicles, mucosal epithelium, retina or uvea of the eye, leptomeninges, or inner ear, but it typically develops on the skin.
The incidence of cutaneous melanoma varies throughout the world. In Western countries, cutaneous melanoma is a relatively common malignancy, especially in populations with lighter skin colors. According to the International Agency for Research on Cancer, its incidence is highest in Queensland, Australia1 but is also high in Auckland, New Zealand.2 In the USA, melanoma is the fifth most commonly diagnosed cancer.3 The incidence is low in Asian populations,4 but there is a definite upward trend in Koreans.5 This brief review is meant to emphasize the characteristics of cutaneous melanoma in Asians, to allow for better rates of early detection of the disease and thus its timely treatment.
According to the WHO classification, cutaneous melanoma can be classified by its location, the amount of sun exposure at the affected site, and the histopathological features of the tumor.6 There are four main subtypes: lentigo maligna melanoma (LMM), superficial spreading melanoma (SSM), acral lentiginous melanoma (ALM), and nodular melanoma (NM). LMM appears mostly on the face or arms of elderly patients (age >70 years) who have been exposed to sunlight throughout their life. It starts as an irregular brown patch and gradually enlarges to form a warty protruding, bleeding, ulcerative lesion. Histopathologically, LMM is characterized by lentiginous proliferation of atypical cells along the basal layer of flattened epidermis and hair follicles, and marked solar elastosis in the dermis (Fig. 1A, B). SSM is the most common subtype in Caucasians and primarily occurs in middle-aged patients (30-50 years old). The tumor may develop on the chest, abdomen, back, upper arms and buttocks: areas that are more likely to be exposed to sunlight. It starts as an irregular patch of variable color, including brown, red, blue-gray, pink, or black, and is commonly associated with pre-existing nevi. The history of SSM often describes a lesion that slowly changes over months or years, which accounts for its common misdiagnosis as a seborrheic keratosis or nevus. Histopathologic features of SSM show extensive pagetoid spread of epithelioid cells in the epidermis (Fig. 1C). ALM differs in its frequency among ethnic groups. The most common sites are the palms of the hands, soles of the feet, and the nails. The typical clinical appearance of ALM is a brown-black lesion with irregular borders. NM mostly occurs on the trunk, but any site may be affected. The lesions often lack an apparent radial growth pattern and reveal deep nodular vertical growth of melanoma cells in the dermis (Fig. 1D), which accounts for the poor prognosis compared with other melanoma subtypes.
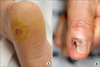
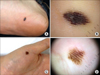
In Asians, SSM is less common than ALM, which is the most common subtype. ALM was first described by Reed in 1976.7 In a Korean study of melanoma, ALM accounted for >70% of the cases.8 The most frequently affected sites are the palms, soles, and nails (Fig. 2). On the sole, the heel is the most common site from the results of anatomic mapping.9 Also, weight-bearing portions of the sole represent the more common sites of ALM. Subungual melanoma is a variant of ALM and generally arises from the nail matrix as a widening, brown-to-black-pigmented lesion on the nail plate with or without nail fragility. The presence of pigmentation involving the nail spreading onto the skin beyond proximal or lateral nail folds is referred as Hutchinson's nail sign, which is the diagnostic clue for subungual melanoma (Fig. 3).10 Based on recent histopathologic analysis of 149 acral melanomas of the palms, soles, and nails in Koreans, the most common type was ALM representing 86.6% (129/149), while NM (19/149, 12.8%) was the second most common type.9 On the other hand, SSM was very rare (1/149, 0.7%) among acral melanomas. Histopathologically, ALM shows only scattered single cell proliferation of hyperchromatic cells surrounded by a clear halo along the basal layer in the very early stages of melanoma in situ (Fig. 4A). Those features always pose a diagnostic challenge, and therefore, a means of clinicopathologic correlation is important to make a diagnosis of ALM. Over time, melanoma cells continuously proliferate along the dermo-epidermal junction and invade into the dermis (Fig. 4B). Most cells are hyperchromatic cells or spindle cells with prominent dendrites in melanoma in situ where prominent dendrites are well represented with HMB45 (Fig. 4C), but mixed cell types of heavily pigmented epithelioid and spindle cells in invasive ALM (Fig. 4D). Inflammatory cells are frequently infiltrated, and ulceration is also common in invasive ALM. Symptoms of ALM are mostly nonexistent, but may include pain, bleeding, and ulceration as melanoma aggravates. The lesion may have been present for several years, although in some patients it is noticed only after trauma or bleeding. ALM may be misdiagnosed as a more benign disease. Soon et al.11 reported that up to one-half of ALMs are initially diagnosed as warts, ulcers, hematomas, foreign bodies, or fungal infections. Amelanotic acral melanomas are especially easy to misdiagnose as benign diseases, such as poroma, unhealing wound, or granuloma pyogenicum (Fig. 5).12 However, a differential diagnoses of a de novo acral lesion, regardless of its color, must include ALM.
The etiology of melanoma is still unclear, but several risk factors have been identified, including sun exposure, genetics, a lighter-skinned phenotype, and pre-existing giant congenital melanocytic nevi (GCMN).1314
In Caucasians, SSM and LMM are the most common subtypes of melanoma and both are generally related to UV radiation. Intermittent sun exposure and sunburns were shown to be significantly and positively related to the incidence of SSM, while individuals with chronic sun exposure had a higher rate of LMM development.15 ALM is the most common subtype of melanoma in Asians who are not chronically exposed to UV radiation. Moreover, in Asians, the role of UV radiation in the development of melanoma is still unclear.1617 Some studies have examined the incidence of melanoma in geographic locations with a high level of sun exposure. Eide and Weinstock18 evaluated data from US Surveillance, Epidemiology, and End Results, collected over 10 years, and divided the patients into racial subgroups, including blacks, Hispanics, Asian/Pacific Islanders, Caucasians, and Native Americans. There was no significant correlation between either the UV index or the latitude and melanoma, except in Caucasians. This finding suggests that mechanisms other than sun exposure contribute to the pathogenesis of melanoma in other racial groups. Thus, in these populations the etiology remains to be determined. In one Korean study, ALM was found to occur frequently on the thumbnail of the finger, toenail of the large toe, and weight-bearing portion of the soles, suggesting the chronic pressure or physical stress might be associated with ALM development.9 Even though only eight out of 177 patients (8.5%) recalled their exact trauma history, trauma or chronic pressure was raised as one of the possible causes of ALM.9
Individuals with a tendency to become sunburned, who have Fitzpatrick skin phototype I-III, blond or red hair, blue or green eyes, and skin that is resistant to pigmentation and prominently freckled have an increased risk of melanoma.1920 By contrast, melanoma occurs much less frequently in individuals with a skin phototype from IV-VI. This suggests a protective role for melanin in preventing the development of melanoma. Asians mostly show skin phototypes III or IV, therefore, they develop UV-associated melanomas less frequently.
Both the number of nevi and their quality (typical vs. atypical) have been implicated in the risk of melanoma. Individuals with >50 typically appearing nevi or with atypical nevi tend to be at risk.21 Dysplastic nevus doubles the risk of melanoma, and having >10 atypical nevi is associated with a 12-fold higher risk.22 GCMN are well-known risk factors for melanoma development, especially in children. In a Korean nationwide study, two cutaneous melanomas and one meningeal melanoma were detected in 131 Koreans with GCMN.14 Therefore, people with melanocytic nevi should be aware of the increased risk of melanoma and undergo regular check-ups by a dermatologist.
Like most other diseases, melanoma has a familial component. Familial melanoma accounts for 10-15% of all melanoma cases. Having a first-degree relative with melanoma increases an individual's risk of melanoma by 35- to 70-fold in Western countries. Even though familial history of melanoma in Koreans have not been studied, the role of familial history on melanoma development does not not seem to be high in Asians. Accordingly, the genetic basis of melanoma is of great interest to researchers and clinicians. Recently, genetic changes have been increasingly detected in cutaneous melanomas, and the most often implicated genes are BRAF, NRAS, and KIT, with BRAF mutations characteristic of SSM patients. These BRAF-mutated patients are also likely to have intermittently sun-damaged skinrather than chronically sun-damaged skin, which correlates with intermittent sun exposure as a significant risk factor for SSM.23 ALM is not associated with sun exposure, and BRAF mutations in these patients are not frequent. Rather in ALM and LMM, KIT mutations and increased copy numbers are detected frequently. Some of these patients may have a good response to treatment with tyrosine kinase inhibitors such as imatinib.24 In Asians, KIT aberrations, such as mutations and increased copy numbers, are the most frequent changes in cutaneous melanoma of Chinese and Japanese populations.2526 In Koreans, ALM is the most common subtype, and KIT mutations and increased copy numbers are mostly associated with ALM.27
The ABCDE (asymmetry, border irregularity, color variegation, diameter, and elevation or evolution) criteria for melanoma are a useful guide for its detection. Melanomas in hidden anatomic locations have been related to thicker tumors at diagnosis, due to their later detection.28 These findings highlight the importance of a thorough skin examination in high-risk patients. In this group, clinical photography surveillance has a higher sensitivity in melanoma detection than does surveillance by a patient or physician.29
Dermoscopy is a noninvasive technique that provides a 10-fold magnification of a lesion. It has a high sensitivity and specificity for the diagnosis of melanoma, without the need for an invasive technique such as biopsy. Among the many different diagnostic algorithms that have been introduced, pattern analysis is the most widely used as it provides an overall impression. Multiple dermoscopic patterns have been associated with specific tumor types. For example, for lesions on the palms and soles, ALM typically shows a "parallel ridge pattern" while acral melanocytic nevi shows a "parallel furrow pattern" (Fig. 6).30 These characteristic dermoscopic findings are very helpful for differentiating melanomas from melanocytic nevi on the palms and soles.
Histopathologic evaluation offers the highest sensitivity and specificity in the diagnosis of melanoma. The histopathologic findings of cutaneous melanoma include an asymmetric tumor with tumor cells occurring both singly and in nests along the dermoepidermal junction, as well as pagetoid spreading on the radial growth phase, and then forming invasive dermal tumor nodules on the vertical growth phase. Adequate biopsy specimens are critical, and partial excisional biopsies or multiple specimens are preferred over punch biopsies because melanoma lesions are usually large. Subungal lesions pose a unique challenge to dermatologists because of the difficulty in biopsying the lesions, as an adequate sample requires a large elliptical incision over the nail matrix. Despite the confirmed value of a histologic diagnosis, the findings may be ambiguous, especially for early stages of ALM. In these cases, immunohistochemical analysis of S-100, HMB45, and Ki-67 is often informative. In a study by Kim et al,31 80% of ALMs stained with 80% of ALMs were positive for HMB45 stain. However, HMB45 stains frequently show focal positive or negative in amelanotic acral melanomas.12 Therefore, amelanotic acral melanomas pose diagnostic challenges both clinically and histoapthologically.
The accurate staging of melanoma is essential for its treatment. In the widely used American Joint Committee on Cancer Staging System, five stages are recognized based on the degree of invasion: stage 0 (in situ disease), stage 1-2 (localized to the skin), stage 3 (lymph node metastasis), and stage 4 (distant metastatic disease).32 In tumor staging, the thickness of the lesion is measured from the top of the granular layer of the epidermis or the base of ulceration to the greatest depth of tumor invasion (Breslow thickness). The sub-T stage is classified as <1 mm, 1-2 mm, 2-4 mm, and >4 mm. The Breslow thickness is the most important prognostic factor for survival in patients with stages 1-2 of the disease: increasing Breslow thickness is associated with decreased survival rates. Laboratory tests, ultrasonography, computed tomography, magnetic resonance imaging, and positron emission tomography are imaging options for the detection of metastasis. Occult micrometastases in regional lymph nodes that are not detected by various imaging studies are best discovered using a sentinel lymph node biopsy (SLNB). Indication for a SLNB is a Breslow thickness >1 mm or a thickness <1 mm in the presence of mitoses.33 In a large cohort study of 101,229 non-Caucasian (Asians, Hispanics, African Americans, and Pacific Islanders) melanoma patients treated in 2012, nonwhite females were significantly more likely to have the disease in thelaterstages at diagnosis.13 Therefore, in Asian populations, public education regarding melanoma prevention and detection is essential.
The standard of treatment for primary cutaneous melanoma was wide local excision with 5-cm tumor-free margins or, in cases of ALM, amputation of the invaded extremity. However, several large studies have failed to reveal a difference in survival rates between wide local excision and excision depending on the Breslow thickness.343536 Hence, the current recommendation for primary cutaneous melanoma is excision according to the Breslow thickness. For example, for melanoma in situ, the margins are 0.5-1 cm; for invasive melanomas, clinical margins should be 1 cm for tumors with a Breslow thickness <1 mm, 1-2 cm for tumors 1-2 mm thick, depending on the anatomy, and 2 cm for melanomas >2 mm thick. However, physicians should take into account current therapeutic trends, the involved anatomic site, and the possibility of micrometastasis in treating each patient. Usually, all of the subcutaneous fat in the margin is removed to achieve the recommended surgical depth.
As mentioned above, SLNB procedure has many advantages to identify micrometastatic lymph nodes. By Multicenter Selective Lymphadenectomy Trial (MSLT-I), SLNB is a safe, low-morbidity procedure for staging in early melanoma.37 Moreover, the 5-year survival was significantly different between patient groups with immediate lymphadenectomy based on a positive SLNB versus those who underwent lymphadenectomy after the metastatic lymph nodes became palpable (72% vs. 52%).38 Among patients with a positive SLNB, 20% showed evidence of non-SLN metastases, discovered during a complete lymph node dissection (CLND).39 While CLND is associated with side effects such as lymphedema, it is recommended for patients with a positive SLN.
In melanoma-prevalent countries, 62.5% of melanoma patients have stage 0 or 1 disease, and another 23.1% have stage 2 disease; thus, melanoma is detected during the early stages in the majority of patients.40 However, in a study from Taiwan, only 40% of melanoma patients had stage I or II disease.41 Given the high risk of relapse of disease for patients advanced past stage II, such as a primary melanoma with a thickness >4 mm and characterized by ulceration or nodal invasion, adjuvant therapy is recommended as an additional treatment. Currently, interferon-α (IFN-α) for patients with stage 3 melanoma and interleukin-2 (IL-2) for those with stage 4 melanoma are the only adjuvant therapies approved by the U.S. Food and Drug Administration (FDA). Both drugs result in significant improvements in disease-free survival.4243 However, there are significant toxicities associated with IFN-α and IL-2, such as flu-like symptoms in almost all patients and myelosuppression; these patients may require a dose reduction. Nonetheless, both therapies reportedly improved the quality of life of the treated patients.44
In melanoma, chemotherapy in not considered curative, but does play a role in the palliative treatment of patients with metastatic melanoma. Dacarbazine is the most active single chemotherapeutic agent, with temporal response rates of 15-25%.45 When combined with tamoxifen, cisplatin, carmustine, or vinblastine, the response rate achieved by dacarbazine is even higher, although there was no effect on disease progression.4647 Patients with brain metastases are typically treated with temozolomide or radiation therapy.48 Despite the resistance of melanomas to radiation therapy, it is used to treat patients with brain or bone metastases for the purpose of reducing pain. Unresectable melanomas are also treated with radiation therapy.
Molecular-targeted therapies have yielded promising results. The above-mentioned KIT inhibitor imatinib has been used effectively in patients with melanomas harboring KIT mutations.49 Vemurafenib and dabrafenib have also produced significant responses in melanomas with BRAF mutations. The FDA in the USA has approved seven novel agents since 2011, such as peginterferon-alfa-2b (2011), BRAF-inhibitors (vemurafenib 2011, dabrafenib 2013), anti-CTLA-4 antibody (ipilimumab 2011), MEK-inhibitors (trametinib 2013), and anti-PD1 antibodies (nivolumab 2014, pembrolizumab 2014), intended to be used in the most advanced cases of melanoma.50 These and other immunotherapeutic agents are expected to play increasingly important therapeutic roles. However, in most countries, including Korea, these drugs are extremely expensive, and their cost is not covered by health insurance. This will hopefully change, and further therapeutic results of those immunotherapeutic agents on Asian melanomas will be accumulated in the near future.
Melanoma is an uncommon cutaneous carcinoma, but it has a high risk of metastasis and a high mortality rate. In Asians, ALM is the most common type of melanoma, but it is usually detected during later stages than are other melanoma types. The education of patients and physicians is critical to ensuring timely melanoma surveillance, including of sun-protected areas of the body. The ABCDE criteria is useful in the early self-detection and -assessment of melanoma. Physicians should hone their skills to avoid the misdiagnosis of melanoma as a benign lesion, especially in patients with amelanotic melanoma. Better-informed patients and physicians will allow for dramatic improvement in the diagnosis of melanoma and significant reductions in melanoma-related mortality.
Figures and Tables
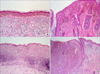 | FIG. 1Histopathologic features of cutaneous melanoma. Lentigo maligna melanoma is characterized by (A) lentiginous proliferation of atypical cells along the basal layer of flattened epidermis and marked solar elastosis in the dermis (H&E, ×100), and (B) proliferated along the hair follicles (H&E, ×100). (C) Superficial spreading melanoma show many pagetoid spread of epithelioid cells in the epidermis (H&E, ×100). (D) Nodular melanoma reveal deep nodular vertical growth of melanoma cells in the dermis without radial growth phase (H&E, ×40). |
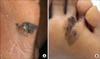 | FIG. 2Acral lentiginous melanoma. (A) Black irregular large patch on the left sole. (B) Brownish to black irregular patch on the left forefoot. |
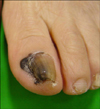 | FIG. 3Hutchinson's nail sign. Black pigmented patch spreading onto the skin beyond the nail folds on the left great toenail. |
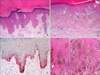 | FIG. 4Histopathologic findings of acral lentiginous melanoma. (A) In the very early stages of melanoma in situ, there are only scattered single cell proliferation of hyperchromatic cells surrounded by a clear halo along the basal layer (H&E, ×100). (B) Over time, melanoma cells are continuously proliferated along the dermo-epidermal junction and invade into the dermis (H&E, ×100). (C) HMB45 immunostain reveals spindle cells with prominent dendrites in melanoma in situ (original magnification, ×100). (D) There are invading mixed cell types of heavily pigmented epithelioid and spindle cells (H&E, ×40). |
References
1. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. Vol. IX. Lyon: IARC Scientific Pubulications;2007.
2. Sneyd M, Cox B. The control of melanoma in New Zealand. N Z Med J. 2006; 119:U2169.
4. Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009; 28:96–102.

5. Bellew S, Del Rosso JQ, Kim GK. Skin cancer in asians: part 2: melanoma. J Clin Aesthet Dermatol. 2009; 2:34–36.
6. Elder DE, Elenitasas R, Murphy GF, Xu X. Benign pigmented lesions and malignant melanoma. In : Elder DE, Elenitasas R, Rosenbach M, Murphy GF, Rubin AI, Xu X, editors. Lever's Histo-Pathology of the Skin. 11th ed. Philadelphia: Wolters Kluwer;2014. p. 853–968.
7. Reed RJ. New concepts in surgical pathology of the skin. New York: Wiley;1976. p. 89–90.
8. Lee MW, Koh JK, Kwon KS, Kim NI, Kim SW, Kim SN, et al. Clinical and histopathological study of cutaneous melanoma in Korea. Korean J Dermatol. 2003; 41:43–47.
9. Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol. 2013; 149:1281–1288.

10. Yun SJ, Kim SJ. Images in clinical medicine. Hutchinson's nail sign. N Engl J Med. 2011; 364:e38.
11. Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. J Am Acad Dermatol. 2003; 48:183–188.

12. Choi YD, Chun SM, Jin SA, Lee JB, Yun SJ. Amelanotic acral melanomas: clinicopathological, BRAF mutation, and KIT aberration analyses. J Am Acad Dermatol. 2013; 69:700–707.

13. Park SL, Le Marchand L, Wilkens LR, Kolonel LN, Henderson BE, Zhang ZF, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res (Phila). 2012; 5:423–434.

14. Yun SJ, Kwon OS, Han JH, Kweon SS, Lee MW, Lee DY, et al. Clinical characteristics and risk of melanoma development from giant congenital melanocytic naevi in Korea: a nationwide retrospective study. Br J Dermatol. 2012; 166:115–123.

15. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997; 73:198–203.

16. Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003; 17:1177–1179.

17. Sheehan JM, Cragg N, Chadwick CA, Potten CS, Young AR. Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J Invest Dermatol. 2002; 118:825–829.

18. Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations--US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol. 2005; 141:477–481.

19. Veierød MB, Weiderpass E, Thörn M, Hansson J, Lund E, Armstrong B, et al. A prospective study of pigmentation, sun exposure, and risk of cutaneous malignant melanoma in women. J Natl Cancer Inst. 2003; 95:1530–1538.

20. Holly EA, Aston DA, Cress RD, Ahn DK, Kristiansen JJ. Cutaneous melanoma in women. II. Phenotypic characteristics and other host-related factors. Am J Epidemiol. 1995; 141:934–942.

21. Garbe C, Büttner P, Weiss J, Soyer HP, Stocker U, Krüger S, et al. Associated factors in the prevalence of more than 50 common melanocytic nevi, atypical melanocytic nevi, and actinic lentigines: multicenter case-control study of the Central Malignant Melanoma Registry of the German Dermatological Society. J Invest Dermatol. 1994; 102:700–705.

22. Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997; 277:1439–1444.

23. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005; 353:2135–2147.

24. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006; 24:4340–4346.

25. Kong Y, Si L, Zhu Y, Xu X, Corless CL, Flaherty KT, et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res. 2011; 17:1684–1691.

26. Ashida A, Takata M, Murata H, Kido K, Saida T. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009; 124:862–868.

27. Jin SA, Chun SM, Choi YD, Kweon SS, Jung ST, Shim HJ, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013; 133:579–582.

28. Nagore E, Oliver V, Moreno-Picot S, Fortea JM. Primary cutaneous melanoma in hidden sites is associated with thicker tumours - a study of 829 patients. Eur J Cancer. 2001; 37:79–82.

29. Weinstock MA. Cutaneous melanoma: public health approach to early detection. Dermatol Ther. 2006; 19:26–31.

30. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011; 38:25–34.

31. Kim YC, Lee MG, Choe SW, Lee MC, Chung HG, Cho SH. Acral lentiginous melanoma: an immunohistochemical study of 20 cases. Int J Dermatol. 2003; 42:123–129.

32. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206.

33. Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004; 351:998–1012.

34. Khayat D, Rixe O, Martin G, Soubrane C, Banzet M, Bazex JA, et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer. 2003; 97:1941–1946.

35. Krown SE, Chapman PB. Defining adequate surgery for primary melanoma. N Engl J Med. 2004; 350:823–825.

36. Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg. 1991; 126:438–441.
37. Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, Glass EC, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005; 242:302–311. discussion 311-3.
38. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006; 355:1307–1317.

39. Sabel MS, Griffith K, Sondak VK, Lowe L, Schwartz JL, Cimmino VM, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005; 201:37–47.

40. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998; 83:1664–1678.

41. Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC, et al. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma Res. 2004; 14:537–541.

42. Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000; 18:2444–2458.

43. Acquavella N, Kluger H, Rhee J, Farber L, Tara H, Ariyan S, et al. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008; 31:569–576.

44. Kilbridge KL, Weeks JC, Sober AJ, Haluska FG, Slingluff CL, Atkins MB, et al. Patient preferences for adjuvant interferon alfa-2b treatment. J Clin Oncol. 2001; 19:812–823.

45. Treisman J, Garlie N. Systemic therapy for cutaneous melanoma. Clin Plast Surg. 2010; 37:127–146.
46. Del Prete SA, Maurer LH, O'Donnell J, Forcier RJ, LeMarbre P. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep. 1984; 68:1403–1405.
47. Legha SS, Ring S, Papadopoulos N, Plager C, Chawla S, Benjamin R. A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine, and dacarbazine (CVD) for metastatic melanoma. Cancer. 1989; 64:2024–2029.

48. Hofmann M, Kiecker F, Wurm R, Schlenger L, Budach V, Sterry W, et al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol. 2006; 76:59–64.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download