Abstract
123I-meta-iodobenzylguanidine (MIBG) has become widely applied in Japan since its introduction to clinical cardiology and neurology practice in the 1990s. Neurological studies found decreased cardiac uptake of 123I-MIBG in Lewy-body diseases including Parkinson's disease and dementia with Lewy bodies. Thus, cardiac MIBG uptake is now considered a biomarker of Lewy body diseases. Although scintigraphic images of 123I-MIBG can be visually interpreted, an average count ratio of heart-to-mediastinum (H/M) has commonly served as a semi-quantitative marker of sympathetic activity. Since H/M ratios significantly vary according to acquisition and processing conditions, quality control should be appropriate, and quantitation should be standardized. The threshold H/M ratio for differentiating Lewy-body disease is 2.0-2.1, and was based on standardized H/M ratios to comparable values of medium-energy collimators. Parkinson's disease can be separated from various types of parkinsonian syndromes using cardiac 123I-MIBG, whereas activity is decreased on images of Lewy-body diseases using both 123I-ioflupane for the striatum and 123I-MIBG. Despite being a simple index, the H/M ratio of 123I-MIBG uptake is reproducible and can serve as an effective tool to support a diagnosis of Lewy-body diseases in neurological practice.
Meta-iodobenzylguanidine (MIBG) is an analogue of noradrenaline (norepinephrine) that is uptaken, stored and released in sympathetic nerve terminals. When radioactively labeled with Iodine-123 (123I), MIBG has the unique ability to reflect sympathetic nerve activity, integrity and disturbances under various pathological conditions that cannot be determined from anatomical findings of current high-resolution computed tomography and magnetic resonance imaging. Since the Japanese Ministry of Welfare (currently the Ministry of Health, Labor and Welfare) approved 123I-MIBG in 1992 for clinical cardiology, further indications have included acute coronary syndrome, repetitive ischemic episodes, arrhythmia and heart failure. The Japanese Circulation Society then added the assessment of severity, treatment effects and prognosis of heart failure to the list of applications.12 Neurology applications started with Parkinson's disease (PD) in the mid-1990s, and was subsequently followed by assessment of dementia with Lewy bodies (DLB), pure autonomic failure (PAF) and related movement disorders. Parkinson's disease is a progressive disorder of the nervous system that affects movement. Symptoms of tremors, stiffness and slow movement that are characteristic can be improved to some degree with appropriate medications. Even with consensus diagnostic criteria, however, precise diagnosis and differentiation between PD and other types of neurodegenerative Parkinsonism present challenges for neurology specialists. The second most common form of progressive dementia after Alzheimer's disease is DLB, and it is accompanied by a progressive decline in mental abilities.3 Memory impairment may not necessarily be prominent in the early stages but it usually becomes evident as the disease progresses. Visual hallucinations, fluctuations in alertness and attention and features of Parkinsonism might be associated. This article focuses on the neurological applications of 123I-MIBG, with focus on PD and DLB. The methodology of 123I-MIBG and need for standardization are also discussed, since 123I-MIBG quantitation is quite complicated due to several technical factors.45
A dose of 111 MBq of MyoMIBG (FUJIFILM RI Pharma Co. Ltd., Tokyo, Japan) is injected intravenously for clinical studies using 123I-MIBG.6 No specific patient preparation is used in Japan other than avoiding medications such as tricyclic antidepressants that significantly interfere with myocardial 123I-MIBG uptake. Scintigraphic images including anterior planar view with 256×256 matrices are usually acquired within 3-10 minutes. Early images are usually acquired between 15 and 30 minutes, and late images are acquired at three to four hours after administration. Data are acquired with energy centered at 159 keV with a 15% or 20% window. The recommended collimator is a medium-energy (ME) type in European guidelines7, but low-energy (LE) types such as low-energy high-resolution (LEHR) and low-energy general-purpose (LEGP) collimators have also been widely applied. The H/M ratio is significantly influenced by collimator selection89 and this is addressed later in this article. Single-photon emission computed tomography (SPECT) imaging can also differentiate Lewy body diseases from other causes of a decrease in cardiac 123I activity in images. Thus, SPECT imaging can be used for scoring defects similarly to perfusion defects that are commonly used to determine the size of a defect or the extent of ischemia.10
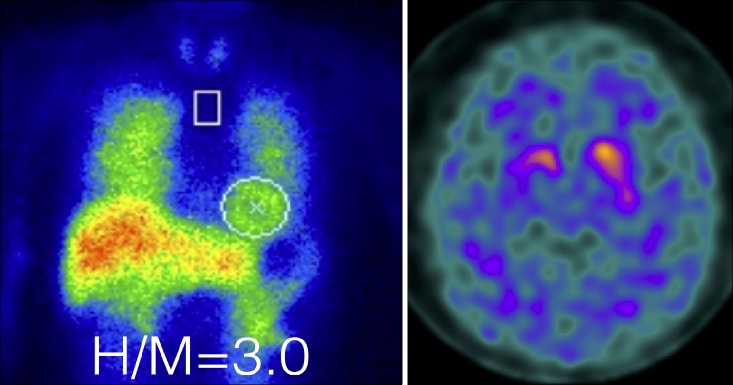
Regions of interest (ROI) are placed on the heart following the shape of the heart, and a rectangular ROI is placed in the upper mediastinum in the anterior view. Although recommended ROI settings differ somewhat, the differences are minor and we have used a semiautomatic method to set ROI (smartMIBG software, developed under collaboration with FUJIFILM RI Pharma, Tokyo, Japan).11 This software points into the center of the heart, and a circular ROI on the heart and a rectangular ROI on the upper mediastinum are automatically determined (Fig. 1). The H/M ratio is defined as the ratio between the average heart count divided by the average mediastinal count. Late images are acquired over three to four hours, during which the H/M ratio remains relatively stable. Washout rates (WR) can be calculated as:
The mediastinal count is usually subtracted from the heart count as a background, and a 123I decay factor (half-life, 13 h) is used to correct for decay during the three to four hours that elapse after the first image acquisition.12 Although ROI settings are generally considered reproducible, the semiautomatic algorithm applied using smart-MIBG software has significantly improved inter- and intra-observer variations.
Although the method of calculating H/M ratios seems simple, the results are significantly influenced by camera-collimator systems, particularly collimators, which have hampered direct inter-institutional comparisons and universal applications in multicenter studies.1314 Because of the differences in septal penetration of the collimator and down-scatter from high-energy photons of 123I, the H/M ratio is generally lower when calculated using LE, than ME collimators. We have thus proposed correcting collimator factors using a camera-collimator specific conversion coefficient4 that can be determined using a cross-calibration phantom. The conversion coefficient is defined as the measured H/M ratio of the phantom divided by the mathematically calculated reference H/M ratio. For example, conversion coefficients of LEHR, LEGP, low-medium energy general purpose (LMEGP) and ME general-purpose (MEGP) collimators are 0.55, 0.65, 0.83 and 0.88, respectively. Even among the collimators with the same name such as LEHR collimators, specifications for septal thickness, hole length and diameter significantly differ among camera vendors. Specific conversion coefficients should be determined at each institution before 123I-MIBG imaging starts. As such, we recommended conversion of the institutional H/M ratio to the standardized condition with a MEGP collimator (conversion coefficient of 0.88), which is in accordance with European guidelines for MIBG imaging.4 The conversion formula for the standardized H/M ratio is:
0.88/(camera-collimator specific conversion coefficient) ×(institutional measured H/M ratio-1) +1
Fig. 2 shows images of the calibration phantom. The difference in image quality is significant between LE high-resolution and ME low-penetration (MELP) collimators. The background activity in the mediastinum is higher using a LE collimator as shown in the phantom image, which causes underestimation compared with the H/M ratio and an ME collimator.
The Lewy-body disease entity includes PD, DLB and PAF, and Japanese investigators first identified low cardiac 123I-MIBG uptake in patients who had PD with and without autonomic failure during 1994.17 Subsequent Japanese and European studies supported these findings.17181920 The diagnostic accuracy of decreased cardiac 123I-MIBG uptake is relatively high in 80%-90% of patients with PD.21 In contrast, cardiac MIBG uptake is preserved in patients with other related disorders including Parkinsonian syndromes such as multiple system atrophy (MSA), progressive supra-nuclear palsy (PSP), and corticobasal degeneration (CBD). Based on the findings of meta-analyses, the sensitivity and specificity range between 80% and 90% (Fig. 3). Although a potential decrease in MIBG uptake based on complications of cardiac diseases such as coronary artery disease, cardiomyopathy and diabetes mellitus, should be carefully interpreted, a severe defect that involves the whole heart is rare, and SPECT imaging is helpful for differentiation.
From a pathophysiological viewpoint, the decrease in myocardial MIBG uptake reflects the fact that abnormalities are not restricted in the brain, but sympathetic neuronal function is more extensively deranged even at the early stages of Lewy-body diseases.6 In fact, Lewy bodies are widely distributed in the hypothalamus, sympathetic and parasympathetic systems, cardiac plexus, enteric nervous system of the alimentary tract, pelvic plexus and adrenal medulla.
Uptake was also obviously reduced in patients with DLB compared with AD after 2001.2223 The second most common cause of dementia is DLB, and its actual incidence seems underestimated. According to the consensus guidelines of the DLB consortium, fluctuations in alertness and attention, visual hallucinations and idiopathic Parkinsonism are major symptoms. However, differentiation from Alzheimer's disease, the most common type of dementia, is not always straightforward and misdiagnosis can influence subsequent treatment strategies. The consortium guidelines also include diagnostic imaging.24 Dopamine transporter imaging or SPECT imaging using 123I-ioflupane is included as a supportive feature, and SPECT/PET brain perfusion and low cardiac 123I-MIBG uptake are included as suggestive features. Decreased perfusion in the occipital or parietal region of the brain is a characteristic finding and MIBG uptake is reduced in patients with DLB.2526
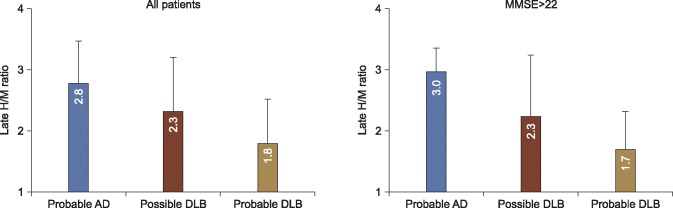
However, since Japanese clinical studies indicate that 123I-MIBG seems to be playing more of a diagnostic role, we started a multi-center study to confirm the ability of cardiac 123I-MIBG uptake to differentiate DLB from AD27 in 133 patients with clinical diagnoses of probable (n=61) and possible (n=26) DLB, and probable AD (n=46). The diagnostic accuracy using early and late H/M ratios was comparable, which means that cardiac uptake was decreased even from the initial uptake. Values for the area under the curve (AUC) of receiver operating characteristics (ROC) were 0.80 and 0.82 based on early and late H/M ratios. The sensitivity and specificity were 77% and 94%, respectively, in a subset of patients with mini-mental state examination (MMSE) scores >22 indicating mild dementia (Fig. 4). This diagnostic accuracy was comparable to previous findings of dopamine transporter imaging, although the data was not available during this multicenter study. Regarding the optimal threshold of the H/M ratio for differentiating DLB from AD, the cutoff value was 2.0 to 2.1 after the HMR was standardized27 for LEHR, LEGP, LMEGP and MEGP collimators. Such standardization of H/M ratios is indispensable to generate reliable information upon which to create a multicenter database.
The optimal threshold for the H/M ratio might differ between its diagnostic applications to neurology and prognostic applications to cardiology. The cut-off H/M ratio for differentiating lethal cardiac events in patients with chronic heart failure is 1.6 in the ADMIRE-HF study,28 1.68 in Japanese pooled 123I-MIBG databases,1529 and 1.75 in a European multicenter MIBG study.30 The appropriate threshold for prognostic evaluation in cardiology might be slightly lower than that in the multicenter study described above27 and the diagnostic threshold of 1.77 for differentiating Lewy body diseases is based on a meta-analysis by King et al.31
Since 123I-ioflupane (DaTSCAN, Nihon Medi-Physics, Tokyo, Japan) was approved in Japan during 2014, its role should be compared with that of 123I-MIBG. 123I-ioflupane accumulates in the striatum with low background activity in healthy persons. Both the caudate nucleus and putamen are clearly visible and comma-shaped. However, accumulation in the putamen is decreased and dot-shaped in patients with suspected Parkinsonian syndromes. It may be used to help differentiate essential tremors from tremors due to PD, MSA, and PSP. The activity of 123I-ioflupane is not decreased in vascular and drug-induced Parkinsonism. Striatal uptake has been quantified using methods such as the specific binding ratio,32 which uses a count ratio between the striatum and background ROI. However, like 123I-MIBG, values for 123I-ioflupane vary considerably depending on the camera collimator system, attenuation and scatter correction and SPECT reconstruction methods. Standardization will be similarly required for 123I-ioflupane studies. In contrast, 123I-MIBG can differentiate PD from Parkinsonian syndromes (Fig. 5) and the activity of both 123I-ioflupane and 123I-MIBG is decreased in patients with Lewy body diseases. Therefore, appropriate use of both methods should be considered according to clinical status and study purpose.
Imaging with 123I-MIBG is a good adjunctive tool for a diagnosis of Lewy body disease. In fact, the role of imaging might be limited for patients with typical symptoms, but the ability to accurately diagnose borderline cases remains a challenge even for neurologists. These imaging modalities could be helpful as a diagnostic tool that non-neurologists can use to refer patient management to more specialized neurologists. 123I-MIBG imaging might also be effective for obtaining an early diagnosis and reaching a confident diagnosis, which would lead to optimal patient management.
ACKNOWLEDGEMENTS
K. Nakajima participates in a collaborative research project with FUJIFILM RI Pharma Co. Ltd. to develop smartMIBG software. This work was partly supported by Grants-in-Aid for Scientific Research in Japan (PI: Kenichi Nakajima). The authors thank Norma Foster for editorial assistance.
References
1. Tamaki N. Guidelines for clinical use of cardiac nuclear medicine (JCS 2005). Circ J. 2005; 69(Suppl IV):1125–1202.
2. Nakajima K, Nakata T. Cardiac 123I-MIBG Imaging for Clinical Decision Making: 22-Year Experience in Japan. J Nucl Med. 2015; 56(Suppl 4):11S–19S. PMID: 26033897.
3. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006; 9(3 Suppl):417–423. PMID: 16914880.
4. Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014; 21:970–978. PMID: 24942608.
5. Slomka PJ, Mehta PK, Germano G, Berman DS. Quantification of I-123-meta-iodobenzylguanidine heart-to-mediastinum ratios: not so simple after all. J Nucl Cardiol. 2014; 21:979–983. PMID: 25005347.
6. Nakajima K, Yoshita M, Matsuo S, Taki J, Kinuya S. Iodine-123-MIBG sympathetic imaging in Lewy-body diseases and related movement disorders. Q J Nucl Med Mol Imaging. 2008; 52:378–387. PMID: 19088692.
7. Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010; 37:1802–1812. PMID: 20577740.
8. Verberne HJ, Feenstra C, de Jong WM, Somsen GA, van Eck-Smit BL, Busemann Sokole E. Influence of collimator choice and simulated clinical conditions on 123I-MIBG heart/mediastinum ratios: a phantom study. Eur J Nucl Med Mol Imaging. 2005; 32:1100–1107. PMID: 15902438.
9. Nakajima K, Matsubara K, Ishikawa T, Motomura N, Maeda R, Akhter N, et al. Correction of iodine-123-labeled meta-iodobenzylguanidine uptake with multi-window methods for standardization of the heart-to-mediastinum ratio. J Nucl Cardiol. 2007; 14:843–851. PMID: 18022111.
10. Nakajima K, Matsumoto N, Kasai T, Matsuo S, Kiso K, Okuda K. Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med. 2016; 30:188–199. PMID: 26897008.
11. Okuda K, Nakajima K, Hosoya T, Ishikawa T, Konishi T, Matsubara K, et al. Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac Iodine-123 MIBG imaging. J Nucl Cardiol. 2011; 18:82–89. PMID: 21104360.
12. Okuda K, Nakajima K, Sugino S, Kirihara Y, Matsuo S, Taki J, et al. Development and validation of a direct-comparison method for cardiac 123I-metaiodobenzylguanidine washout rates derived from late 3-hour and 4-hour imaging. Eur J Nucl Med Mol Imaging. 2016; 43:319–325. PMID: 26298280.
13. Verberne HJ, Habraken JB, van Eck-Smit BL, Agostini D, Jacobson AF. Variations in 123I-metaiodobenzylguanidine (MIBG) late heart mediastinal ratios in chronic heart failure: a need for standardisation and validation. Eur J Nucl Med Mol Imaging. 2008; 35:547–553. PMID: 17922122.
14. Nakajima K, Okuda K, Matsuo S, Yoshita M, Taki J, Yamada M, et al. Standardization of metaiodobenzylguanidine heart to mediastinum ratio using a calibration phantom: effects of correction on normal databases and a multicentre study. Eur J Nucl Med Mol Imaging. 2012; 39:113–119. PMID: 22009380.
15. Nakajima K, Nakata T, Yamada T, Yamashina S, Momose M, Kasama S, et al. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using 123I-metaiodobenzylguanidine imaging. Eur J Nucl Med Mol Imaging. 2014; 41:1673–1682. PMID: 24663289.
16. Nakajima K, Nakata T, Matsuo S, Jacobson AF. Creation of mortalityrisk charts using 123I meta-iodobenzylguanidine heart-to-mediastinum ratio in patients with heart failure: 2- and 5-year risk models. Eur Heart J Cardiovasc Imaging. 2015; [Epub ahead of print]. DOI: 10.1093/ehjci/jev322.
17. Hakusui S, Yasuda T, Yanagi T, Tohyama J, Hasegawa Y, Koike Y, et al. A radiological analysis of heart sympathetic functions with meta-[123I]iodobenzylguanidine in neurological patients with autonomic failure. J Auton Nerv Syst. 1994; 49:81–84. PMID: 7963268.
18. Iwasa K, Nakajima K, Yoshikawa H, Tada A, Taki J, Takamori M. Decreased myocardial 123I-MIBG uptake in Parkinson's disease. Acta Neurol Scand. 1998; 97:303–306. PMID: 9613559.
19. Yoshita M. Differentiation of idiopathic Parkinson's disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci. 1998; 155:60–67. PMID: 9562324.
20. Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H. 123I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999; 67:189–194. PMID: 10406987.
21. Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012; 18:494–500. PMID: 22321865.
22. Yoshita M, Taki J, Yamada M. A clinical role for [123I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer's-type and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2001; 71:583–588. PMID: 11606666.
23. Watanabe H, Ieda T, Katayama T, Takeda A, Aiba I, Doyu M, et al. Cardiac 123I-meta-iodobenzylguanidine (MIBG) uptake in dementia with Lewy bodies: comparison with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001; 70:781–783. PMID: 11385013.
24. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005; 65:1863–1872. PMID: 16237129.
25. Hanyu H, Shimizu S, Hirao K, Kanetaka H, Iwamoto T, Chikamori T, et al. Comparative value of brain perfusion SPECT and [123I]MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2006; 33:248–253. PMID: 16328506.
26. Sakamoto F, Shiraishi S, Yoshida M, Tomiguchi S, Hirai T, Namimoto T, et al. Diagnosis of dementia with Lewy bodies: diagnostic performance of combined 123I-IMP brain perfusion SPECT and 123I-MIBG myocardial scintigraphy. Ann Nucl Med. 2014; 28:203–211. PMID: 24363079.
27. Yoshita M, Arai H, Arai H, Arai T, Asada T, Fujishiro H, et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One. 2015; 10:e0120540. PMID: 25793585.
28. Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010; 55:2212–2221. PMID: 20188504.
29. Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of 123I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013; 6:772–784. PMID: 23845574.
30. Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008; 35:535–546. PMID: 18043919.
31. King AE, Mintz J, Royall DR. Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord. 2011; 26:1218–1224. PMID: 21480373.
32. Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006; 33:1491–1499. PMID: 16858570.
FIG. 1
Semiautomatic method of setting ROI. Circular heart and rectangular mediastinal ROI (height, 30%; width, 10% of the mediastinum) are automatically determined after pointing towards the center of the heart.
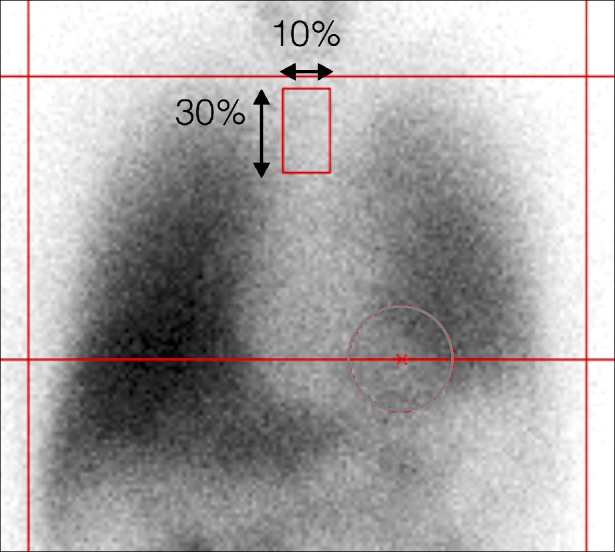
FIG. 2
Typical calibration phantom images with low-energy high-resolution (LEHR) and medium-energy low-penetration (MELP) collimators. Contrast between heart and mediastinal counts is notable in both images. Regions of interest are automatically selected and positioned by smartMIBG software.
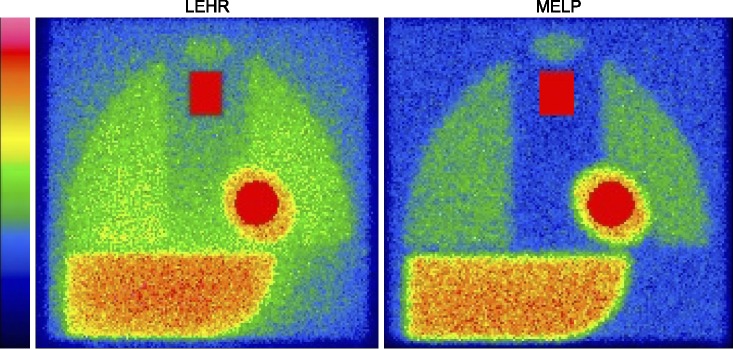
FIG. 3
123I-MIBG H/M ratio in control, Alzheimer's disease (AD), multiple system atrophy (MSA), dementia with Lewy bodies (DLB), Parkinson's disease (PD), and pure autonomic failure (PAF). Regions of interest were positioned using smartMIBG software. H/M ratios are 2.9, 3.7, 3.0, 1.2, 1.7 and 1.2, respectively.
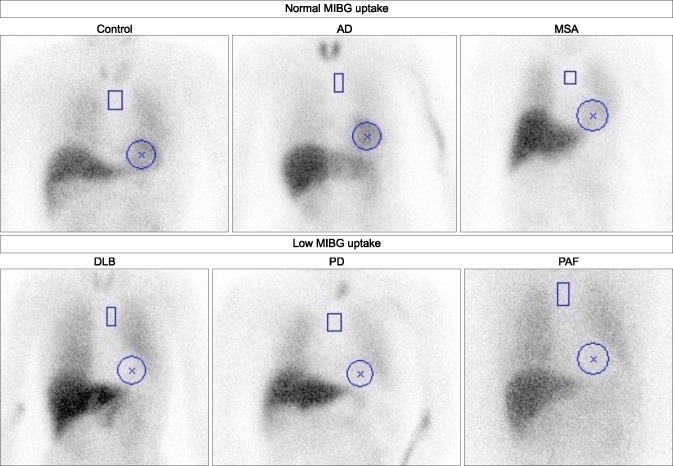
FIG. 4
Late H/M ratios in patients with probable Alzheimer's disease (AD), possible dementia with Lewy bodies (DLB), and probable DLB. Differences are significant among groups (p < 0.0001). Data are from Reference 26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


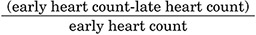
 XML Download
XML Download