Abstract
As humans age, degenerative changes in the arterial structure gradually progress and result in the stiffening of the arteries, which is called arteriosclerosis. Arterial stiffness is now an established risk factor of cardiovascular disease (CVD). This stiffening has adverse effects for both the general population as well as for patients with CVD. Measurements of pulse wave velocity and pulse wave analysis are the two most commonly used methods in the evaluation of arterial stiffness, but these methods just allow indirect measures of arterial stiffness. Echocardiography is the most widely used imaging modality in the evaluation of cardiac structure and function and with recent technical advances, it has become possible to evaluate the structure, function and blood flow hemodynamics of the arteries using echocardiography. In the present review, we will discuss the current status of echocardiography in the evaluation of arterial stiffness, especially focusing on the methodological aspects.
Degenerative changes in the arterial system gradually progress with aging and can be accelerated by certain risk factors.123 Cardiovascular disease (CVD), associated with vascular aging, has become a major cause of death in worldwide. Therefore, to reduce the risk of CVD or CV events the identification and therapeutic modification of the earliest stages of vascular change before those changes develop into overt CVD is essential. Seeing as arterial stiffness is one of the earliest detectable structural and functional changes of the arterial wall, it has been widely studied and proven to be an independent surrogate marker of overt CVD or future CV events.45678910 In the current review, we will discuss the current status of echocardiography in the evaluation of arterial stiffness, especially focusing on methodological aspects.
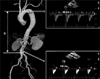
To adapt to hemodynamic stress on the arterial wall with aging, the diameter of the artery is enlarged and the thickness of the arterial wall is increased.123 The degenerative changes of the arterial system with aging are generally categorized into 2 types; atherosclerosis and arteriosclerosis (Fig. 1),11 even though these 2 process may progress simultaneously in many of cases.12 Atherosclerosis is a chronic inflammatory disease, primarily affecting the tunica intima, resulting in smooth muscle cell proliferation and atheromatous plaques.13 Atherosclerosis, therefore, is characterized by arterial stenosis that restricts blood flow through the arterial lumen. On the other hand, arteriosclerosis is a degenerative stiffening of the arterial wall, primarily affecting the tunica media, which is also called arterial stiffness.411 Loss of elastin, the deposition of collagen, and the thickening of the medial layer result in the stiffening of the arterial system. Large population based studies have demonstrated that arterial stiffness is a strong predictor of CVD or CV events not only in the general population, but also in patients with CVD.141516
Systemic, regional, and local arterial stiffness can be measured by various, noninvasive methods. Pulse wave velocity (PWV), a measure of regional aortic stiffness, is the most widely studied and validated noninvasive method because it is a simple, accurate and reproducible method, and a strong predictor of adverse CV outcomes. PWV is now considered to be the gold standard method of measuring arterial stiffness.617 However, despite these advantages, PWV does not reflect the degree of arteriosclerosis in the local arterial wall because it is just an indirect measure of regional arterial stiffness. To overcome or compensate for the limitations of PWV therefore, several alternative methods have been studied to evaluate the degree to which arterial stiffness of the local arterial wall has developed. Echocardiography could be a useful tool for this purpose because it allows not only for the direct visualization of the arterial wall, but also a tool for the measurement of blood flow using the Doppler technique.618
There are 2 prerequisite parameters for evaluating arterial stiffness using echocardiography; the change in blood volume, and the pressure change caused by the volume change.618
The pressure change (ΔP) can be calculated by measuring systolic (SBP) and diastolic blood pressure (DBP); ΔP=SBP-DBP. The volume change can be derived from the diameter change of the artery between systole and diastole which can be easily measured using echocardiography. The diameter change (ΔD) can be calculated by measuring the systolic (SD) and diastolic diameter (DD) of the arteries; ΔD=SD-DD.
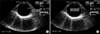
To evaluate aortic stiffness, aortic diameters can be measured by M-mode tracing of the ascending aorta at the level of 3-4 cms above the aortic valve from the parastenal long axis view during transthoracic echocardiography (Fig. 2A). In the case of transesophageal echocardiography (TEE), aortic diameters can be measured by M-mode tracing of the descending thoracic aorta at each level (Fig. 2B). To evaluate arterial stiffness of the carotid artery, the diameter changes can be measured by M-mode tracing of the mid-portion of the common carotid artery (Fig. 2C).18 After the acquisition of the 2 prerequisite parameters, several useful indices of local arterial stiffness can be calculated using the following formula18;
1) Arterial diameter change (mm)=SD-DD
2) Arterial strain=(SD-DD)/DD
3) Elastic modulus E(p)=(SBP-DBP)/strain
4) Arterial stiffness index β=Ln (SBP/DBP)/strain (Ln: natural logarithm)
5) Arterial distensibility=(2×strain)/(SBP-DBP)
Noninvasively calculated aortic stiffness index β showed a strong correlation with the invasive measurements of arterial stiffness19, and aortic stiffness measured by aortic strain, distensibility, and the stiffness index β which is associated with cerebral infarction20 and an independent predictor of the progression to hypertension in non-hypertensive individuals.21 In the previous study, the authors also demonstrated that aortic distensibility showed a significant negative correlation with PWV.22 In addition to the aortic diameter change, the aortic area change using 2D tracing instead of M-mode tracing was measured and used to calculate the parameters of aortic stiffness (Fig. 3). Aortic distensibility measured by aortic area change showed better correlation with PWV than aortic distensibility measured by aortic diameter change in our study. We posited that aortic area change instead of aortic diameter change would be a better data-point in calculating the parameters of aortic stiffness, because aortic area change can reflect the averaged diameter change in the whole direction of the aorta while the aortic diameter change can reflect the diameter change in a single direction of the aorta. In this respect, 3D echocardiography may be a potentially useful tool for the evaluation of arterial stiffness, 23 but the role of 3D echocardiography in the evaluation of arterial stiffness has not yet been satisfactorily examined until this study.
PWV, a measure of regional arterial stiffness measured by tonometry, is generally accepted as the most simple, non-invasive, robust, and reproducible method to determine arterial stiffness and considered as the gold standard measurement in the current estimation.6
Doppler echocardiography can also be used for PWV measurement.1824 Pulse wave Doppler tracing of 2 given arterial sites and calculating the distance between the 2 given arterial sites is required to calculate PWV. PWV can be calculated as the distance between the 2 arterial sites, divided by the transit time determined by the foot to foot method (Fig. 4). PWV measurement by echocardiography however, has some methodological limitations compared to PW Doppler tracing since it is a sequential measurement of the 2 given arterial sites, instead of a simultaneous measurement, which is all that is allowed for by the currently available commercial echocardiography equipment. Also, there have been limited data on the usefulness of PWV measurement by echocardiography.24
2D speckle tracking echocardiography (STE) is a promising new imaging modality not only in the evaluation of myocardial function, but also in the evaluation of myocardial mechanics.25 STE permits offline measurements of myocardial deformation parameters including strain and strain rate. Some researchers have adopted STE to measure arterial strain as an index of arterial stiffness.26272829 The authors first introduced vector velocity imaging STE to measure circumferential strain (CS) of the descending thoracic aorta obtained by TEE (Fig. 5).22 Peak CS of the aorta showed good correlation with aortic PWV and intima-media thickness of the aorta in our study. Despite of the usefulness of vascular strain analysis in evaluating arterial stiffness, the results of our study cannot be easily applied to clinical practice because TEE is a relatively invasive, uncomfortable procedure. To overcome this limitation, STE of the carotid artery has been studied and has demonstrated that global CS of the carotid artery is a useful tool for the evaluation of arterial stiffness (Fig. 6).2829 In our study, carotid CS, not the conventional carotid stiffness index, shows significant negative correlation with aging and PWV in patients with newly diagnosed, untreated hypertension.29 Carotid CS also shows strong correlation with aging, PWV, and the Framingham risk scores used in the study of Park et al.28
Vascular strain analysis by STE, theoretically, seems to be a promising tool in the evaluation of local arterial stiffness, but STE is still only a research tool at this time because there is a lack of normal reference values or outcome data in large population based studies. Large validation studies will be needed to apply this new imaging technique in evaluating arterial stiffness.
Arterial stiffness is an important predictor of CVD or future CV events both in the general population and in patients with overt CVD. Because echocardiography allows for the direct visualization of the arterial structure, it usually has been used to evaluate arterial stenosis by atherosclerosis. As shown in the current review. On the other hand, echocardiography could also be a promising tool in the evaluation of arterial stiffness, especially in evaluating local stiffness. Despite the potential benefits, the current status of echocardiography in the evaluation of arterial stiffness is still merely a research tool, because there is a lack of large population based studies evaluating CVD or CV outcomes. Large validation studies will be needed to apply this new imaging technique in evaluating arterial stiffness. Clinicians or investigators should select the method that is appropriate for clinical application and/or research. Seeing as there are many different echocardiographic parameters of arterial stiffness, the potential advantages and limitations of each method should be considered before choosing the appropriate method.
Figures and Tables
FIG. 2
Measurement of systolic (dashed line) and diastolic (solid line) arterial diameter in the ascending aorta (A), descending throracic aorta (B), and common carotid artery (C).

FIG. 4
Measurements of pulse wave velocity (PWV) by Doppler echocardiographic recoding of 2 aortic sites. T1: time interval between the peak R wave on electrocardiography and the onset of PW Doppler signal of the descending thoracic aorta, T2: time interval between the peak R wave on electrocardiography and the onset of PW Doppler signal of the abdominal aortic bifurcation. D: distance between the beginning site of the descending thoracic aorta and the just above site of the abdominal aortic bifurcation. PWV can be calculated as (T2-T1)/(D).
References
1. Faconti L, Bruno RM, Ghiadoni L, Taddei S, Virdis A. Ventricular and vascular stiffening in aging and hypertension. Curr Hypertens Rev. 2015; 11:100–109.

2. Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: Part 1 of a 2-part review. Circulation. 2011; 123:1650–1660.
3. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007; 50:1–13.
4. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011; 57:1511–1522.
5. Vlachopoulos C, Alexopoulos N, Stefanadis C. Aortic stiffness: prime time for integration into clinical practice? Hellenic J Cardiol. 2010; 51:385–390.
6. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–2605.

7. London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004; 26:689–699.

8. Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004; 109:184–189.

9. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002; 15:426–444.
10. Izzo JL Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med. 2001; 2:29–34. 37–40.
11. Safar ME. Arterial aging--hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010; 7:442–449.

12. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001; 32:454–460.

14. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014; 63:636–646.
15. Pereira T, Maldonado J, Polónia J, Silva JA, Morais J, Rodrigues T, et al. Participants in the Ediva Project. Aortic pulse wave velocity and HeartSCORE: improving cardiovascular risk stratification. a sub-analysis of the EDIVA (Estudo de DIstensibilidade VAscular) project. Blood Press. 2014; 23:109–115.

16. Imanishi R, Seto S, Toda G, Yoshida M, Ohtsuru A, Koide Y, et al. High brachial-ankle pulse wave velocity is an independent predictor of the presence of coronary artery disease in men. Hypertens Res. 2004; 27:71–78.

17. Rhee MY, Lee HY, Park JB. Measurements of Arterial Stiffness: Methodological Aspects. Korean Circ J. 2008; 38:343–350.

18. Nemes A, Geleijnse ML, Forster T, Soliman OI, Ten Cate FJ, Csanády M. Echocardiographic evaluation and clinical implications of aortic stiffness and coronary flow reserve and their relation. Clin Cardiol. 2008; 31:304–309.

19. Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990; 11:990–996.

20. Yoon HJ, Kim KH, Lee SH, Yim YR, Lee KJ, Park KH, et al. Differences of aortic stiffness and aortic intima-media thickness according to the type of initial presentation in patients with ischemic stroke. J Cardiovasc Ultrasound. 2013; 21:12–17.

21. Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005; 45:426–431.

22. Kim KH, Park JC, Yoon HJ, Yoon NS, Hong YJ, Park HW, et al. Usefulness of aortic strain analysis by velocity vector imaging as a new echocardiographic measure of arterial stiffness. J Am Soc Echocardiogr. 2009; 22:1382–1388.

23. Nemes A, Geleijnse ML, Soliman OI, Anwar AM, Vletter WB, ten Cate FJ. Real-time three-dimensional echocardiography for regional evaluation of aortic stiffness. Eur J Echocardiogr. 2007; 8:161–162.

24. Lee MY, Chu CS, Lee KT, Wu CM, Su HM, Lin SJ, et al. Validation of a new index for estimating arterial stiffness: measurement of the QPV interval by Doppler ultrasound. Clin Cardiol. 2006; 29:345–351.

25. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015; 28:183–193.

26. Oishi Y, Mizuguchi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. A novel approach to assess aortic stiffness related to changes in aging using a two-dimensional strain imaging. Echocardiography. 2008; 25:941–945.

27. Kawasaki T, Fukuda S, Shimada K, Maeda K, Yoshida K, Sunada H, et al. Direct measurement of wall stiffness for carotid arteries by ultrasound strain imaging. J Am Soc Echocardiogr. 2009; 22:1389–1395.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download