1. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003; 289:3095–3105. PMID:
12813115.
2. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013; 34:119–138. PMID:
23514317.
3. Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003; 26:457–494. PMID:
12778843.
4. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006; 163:28–40. PMID:
16390886.
5. Wang SM, Han C, Pae CU. Criticisms of drugs in early development for the treatment of depression: what can be improved? Expert Opin Investig Drugs. 2015; 24:445–453.
6. Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Vilazodone for the treatment of major depressive disorder: focusing on its clinical studies and mechanism of action. Psychiatry Investig. 2015; 12:155–163.
7. Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015; 12:1–8.
8. Pae CU. Desvenlafaxine in the treatment of major depressive disorder. Expert Opin Pharmacother. 2011; 12:2923–2928. PMID:
22098230.
9. Pae CU. Desvenlafaxine: a new antidepressant or just another one? Expert Opin Pharmacother. 2009; 10:875–887. PMID:
19351235.
10. Pae CU. Agomelatine: a new option for treatment of depression? Expert Opin Pharmacother. 2014; 15:443–447. PMID:
24397553.
11. Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. A review of current evidence for vilazodone in major depressive disorder. Int J Psychiatry Clin Pract. 2013; 17:160–169. PMID:
23578403.
12. Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, et al. Vortioxetine: a meta-analysis of 12 short-term, randomized, placebo-controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci. 2015; 40:174–186. PMID:
25350320.
13. Pae CU, Park MH, Marks DM, Han C, Patkar AA, Masand PS. Desvenlafaxine, a serotonin-norepinephrine uptake inhibitor for major depressive disorder, neuropathic pain and the vasomotor symptoms associated with menopause. Curr Opin Investig Drugs. 2009; 10:75–90.
14. Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, et al. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res. 2015; 64:88–98. PMID:
25851751.
15. Pae CU. Evidence-based treatment for depressive disorder. Psychiatry Investig. 2015; 12:278–279.
16. Pae CU, Patkar AA, Jun TY, Lee C, Masand PS, Paik IH. Aripiprazole augmentation for treatment of patients with inadequate antidepressants response. Depress Anxiety. 2007; 24:522–526. PMID:
17111388.
17. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006; 163:1905–1917. PMID:
17074942.
18. Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011; 168:689–701. PMID:
21536692.
19. Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007; 62:1217–1227. PMID:
17588546.
20. Papakostas GI, Fava M. A meta-analysis of clinical trials comparing milnacipran, a serotonin--norepinephrine reuptake inhibitor, with a selective serotonin reuptake inhibitor for the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2007; 17:32–36. PMID:
16762534.
21. Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009; 373:746–758. PMID:
19185342.
22. Dennehy EB, Marangell LB, Martinez J, Balasubramani GK, Wisniewski SR. Clinical and functional outcomes of patients who experience partial response to citalopram: secondary analysis of STAR*D. J Psychiatr Pract. 2014; 20:178–187. PMID:
24847991.
23. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000; 157(4 Suppl):1–45.
24. Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ. World Federation of Societies Biological Psychiatry Task Force on Treatment Guidelines for Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 1: Acute and continuation treatment of major depressive disorder. World J Biol Psychiatry. 2002; 3:5–43. PMID:
12479086.
25. Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008; 22:343–396. PMID:
18413657.
26. Kennedy SH, Lam RW, Cohen NL, Ravindran AV. CANMAT Depression Work Group. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry. 2001; 46(Suppl 1):38S–58S. PMID:
11441771.
27. Connolly KR, Thase ME. If at first you don't succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011; 71:43–64. PMID:
21175239.
28. Köhler S, Unger T, Hoffmann S, Steinacher B, Fydrich T, Bschor T. Comparing augmentation with non-antidepressants over sticking to antidepressants after treatment failure in depression: a naturalistic study. Pharmacopsychiatry. 2013; 46:69–76. PMID:
23093475.
29. Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within-versus across-class switches. Biol Psychiatry. 2008; 63:699–704. PMID:
17919460.
30. Thase ME. Antidepressant combinations: widely used, but far from empirically validated. Can J Psychiatry. 2011; 56:317–323. PMID:
21756445.
31. Pae CU, Han C, Jun TY. Do we need more than one antidepressant for patients with major depressive disorder? Expert Rev Neurother. 2011; 11:1561–1564. PMID:
22014134.
32. Pae CU, Wang SM, Lee SY, Lee SJ. Early switch strategy in patients with major depressive disorder. Expert Rev Neurother. 2012; 12:1185–1188. PMID:
23082734.
33. Poirier MF, Boyer P. Venlafaxine and paroxetine in treatment-resistant depression. Double-blind, randomised comparison. Br J Psychiatry. 1999; 175:12–16. PMID:
10621762.
34. Lenox-Smith AJ, Jiang Q. Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor. Int Clin Psychopharmacol. 2008; 23:113–119. PMID:
18408525.
35. Souery D, Serretti A, Calati R, Oswald P, Massat I, Konstantinidis A, et al. Citalopram versus desipramine in treatment resistant depression: effect of continuation or switching strategies: a randomized open study. World J Biol Psychiatry. 2011; 12:364–375. PMID:
21718212.
36. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002; 51:183–188. PMID:
11822997.
37. Ferreri M, Lavergne F, Berlin I, Payan C, Puech AJ. Benefits from mianserin augmentation of fluoxetine in patients with major depression non-responders to fluoxetine alone. Acta Psychiatr Scand. 2001; 103:66–72. PMID:
11202131.
38. Licht RW, Qvitzau S. Treatment strategies in patients with major depression not responding to first-line sertraline treatment A randomised study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology (Berl). 2002; 161:143–151. PMID:
11981594.
39. Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010; 167:281–288. PMID:
20008946.
40. Blier P, Gobbi G, Turcotte JE, de Montigny C, Boucher N, Hébert C, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009; 19:457–465. PMID:
19345072.
41. Stewart JW, McGrath PJ, Blondeau C, Deliyannides DA, Hellerstein D, Norris S, et al. Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res. 2014; 52:7–14. PMID:
24485847.
42. Philip NS, Carpenter LL, Tyrka AR, Price LH. Augmentation of antidepressants with atypical antipsychotics: a review of the current literature. J Psychiatr Pract. 2008; 14:34–44. PMID:
18212601.
43. Pae CU, Patkar AA. Clinical issues in use of atypical antipsychotics for depressed patients. CNS Drugs. 2013; 27(Suppl 1):S39–S45. PMID:
23709360.
44. Han C, Yeh TL, Kato M, Sato S, Chang CM, Pae CU. Management of chronic depressive patients with residual symptoms. CNS Drugs. 2013; 27(Suppl 1):S53–S57. PMID:
23709362.
45. Han C, Wang SM, Kato M, Lee SJ, Patkar AA, Masand PS, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013; 13:851–870. PMID:
23898855.
46. Pae CU, Forbes A, Patkar AA. Aripiprazole as adjunctive therapy for patients with major depressive disorder: overview and implications of clinical trial data. CNS Drugs. 2011; 25:109–127. PMID:
21254788.
47. Pae CU, Sohi MS, Seo HJ, Serretti A, Patkar AA, Steffens DC, et al. Quetiapine XR: current status for the treatment of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:1165–1173. PMID:
20307622.
48. Mischoulon D, Nierenberg AA, Kizilbash L, Rosenbaum JF, Fava M. Strategies for managing depression refractory to selective serotonin reuptake inhibitor treatment: a survey of clinicians. Can J Psychiatry. 2000; 45:476–481. PMID:
10900529.
49. Gaynes BN, Dusetzina SB, Ellis AR, Hansen RA, Farley JF, Miller WC, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012; 32:114–119. PMID:
22198447.
50. Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009; 23:627–647. PMID:
19594193.
51. Wisniewski SR, Fava M, Trivedi MH, Thase ME, Warden D, Niederehe G, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. Am J Psychiatry. 2007; 164:753–760. PMID:
17475734.
52. Warden D, Rush AJ, Wisniewski SR, Lesser IM, Kornstein SG, Balasubramani GK, et al. What predicts attrition in second step medication treatments for depression?: a STAR*D report. Int J Neuropsychopharmacol. 2009; 12:459–473. PMID:
18611293.
53. Papakostas GI, Petersen TJ, Green C, Iosifescu DV, Yeung AS, Nierenberg AA, et al. A description of next-step switching versus augmentation practices for outpatients with treatment-resistant major depressive disorder enrolled in an academic specialty clinic. Ann Clin Psychiatry. 2005; 17:161–165. PMID:
16433058.
54. Shelton RC. What are the comparative benefits and harms of augmentation treatments in major depression? J Clin Psychiatry. 2015; 76:e531–e533. PMID:
25919851.
55. Han C, Wang SM, Seo HJ, Lee BC, Jeon HJ, Kim W, et al. Aripiprazole augmentation, antidepressant combination or switching therapy in patients with major depressive disorder who are partial- or non-responsive to current antidepressants: a multi-center, naturalistic study. J Psychiatr Res. 2014; 49:75–82. PMID:
24268719.
56. Han C, Wang SM, Kwak KP, Won WY, Lee H, Chang CM, et al. Aripiprazole augmentation versus antidepressant switching for patients with major depressive disorder: A 6-week, randomized, rater-blinded,prospective study. J Psychiatr Res. 2015; 66-67:84–94. PMID:
26013203.
57. Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry. 2015; 76:e487–e498. PMID:
25919841.
58. Jing Y, Guo Z, Kalsekar I, Forbes RA, Hebden T, Thase ME. Dosing patterns of aripiprazole and quetiapine for adjunctive treatment of major depressive disorder (2006-2010). Int Clin Psychopharmacol. 2013; 28:87–90. PMID:
23262644.
59. Hsu YC, Chou YC, Chang HA, Kao YC, Huang SY, Tzeng NS. Dilemma of prescribing aripiprazole under the Taiwan health insurance program: a descriptive study. Neuropsychiatr Dis Treat. 2015; 11:225–232. PMID:
25657586.
60. Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS. Aripiprazole augmentation for major depressive disorder: dosing patterns in a naturalistic treatment setting. Int Clin Psychopharmacol. 2014; 29:116–119. PMID:
24108149.
61. Sagud M, Mihaljević-Peleš A, Begić D, Vuksan-Ćusa B, Kramarić M, Zivković M, et al. Antipsychotics as antidepressants: what is the mechanism? Psychiatr Danub. 2011; 23:302–307. PMID:
21963702.
62. Chen TY, Tzeng NS. Aripiprazole: a dopamine modulator that mimics methylphenidate in producing faster antidepressant effects. Med Hypotheses. 2013; 81:183–185. PMID:
23751312.
63. Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005; 138:228–235. PMID:
15925428.
64. Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002; 955:264–267. PMID:
12419546.
65. Otsuka Pharmaceuticals I. Prescribing information: Aripiprazole 2009.
66. Conway CR, Chibnall JT, Cumming P, Mintun MA, Gebara MA, Perantie DC, et al. Antidepressant response to aripiprazole augmentation associated with enhanced FDOPA utilization in striatum: a preliminary PET study. Psychiatry Res. 2014; 221:231–239. PMID:
24468015.
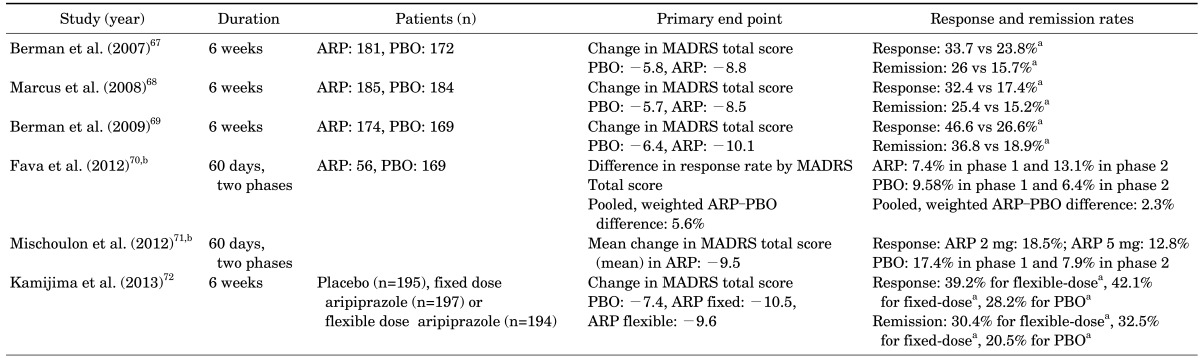
67. Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007; 68:843–853. PMID:
17592907.
68. Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008; 28:156–165. PMID:
18344725.
69. Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009; 14:197–206. PMID:
19407731.
70. Fava M, Mischoulon D, Iosifescu D, Witte J, Pencina M, Flynn M, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom. 2012; 81:87–97. PMID:
22286203.
71. Mischoulon D, Witte J, Levy M, Papakostas GI, Pet LR, Hsieh WH, et al. Efficacy of dose increase among nonresponders to low-dose aripiprazole augmentation in patients with inadequate response to antidepressant treatment: a randomized, double-blind, placebo-controlled, efficacy trial. J Clin Psychiatry. 2012; 73:353–357. PMID:
21939613.
72. Kamijima K, Higuchi T, Ishigooka J, Ohmori T, Ozaki N, Kanba S, et al. ADMIRE Study Group. Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study). J Affect Disord. 2013; 151:899–905. PMID:
24074484.
73. Thase ME, Trivedi MH, Nelson JC, Fava M, Swanink R, Tran QV, et al. Examining the efficacy of adjunctive aripiprazole in major depressive disorder: a pooled analysis of 2 studies. Prim Care Companion J Clin Psychiatry. 2008; 10:440–447. PMID:
19287552.
74. Pae CU, Seo HJ, Lee BC, Seok JH, Jeon HJ, Paik JW, et al. A meta-analysis comparing open-label versus placebo-controlled clinical trials for aripiprazole augmentation in the treatment of major depressive disorder: lessons and promises. Psychiatry Investig. 2014; 11:371–379.
75. Fava M, Wisniewski SR, Thase ME, Baker RA, Tran QV, Pikalov A, et al. Metabolic assessment of aripiprazole as adjunctive therapy in major depressive disorder: a pooled analysis of 2 studies. J Clin Psychopharmacol. 2009; 29:362–367. PMID:
19593176.
76. Lin CH, Lin SH, Jang FL. Adjunctive low-dose aripiprazole with standard-dose sertraline in treating fresh major depressive disorder: a randomized, double-blind, controlled study. J Clin Psychopharmacol. 2011; 31:563–568. PMID:
21869699.
77. Pae CU, Serretti A, Patkar AA, Masand PS. Aripiprazole in the treatment of depressive and anxiety disorders: a review of current evidence. CNS Drugs. 2008; 22:367–388. PMID:
18399707.
78. Citrome L. Number needed to treat: what it is and what it isn't, and why every clinician should know how to calculate it. J Clin Psychiatry. 2011; 72:412–413. PMID:
21450157.
79. Rapaport MH, Gharabawi GM, Canuso CM, Mahmoud RA, Keller MB, Bossie CA, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: Results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology. 2006; 31:2505–2513. PMID:
16760927.
80. Alexopoulos GS, Canuso CM, Gharabawi GM, Bossie CA, Greenspan A, Turkoz I, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008; 16:21–30. PMID:
17928573.
81. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010; 122:39–48. PMID:
20675970.
82. Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, et al. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014; 44:2017–2028. PMID:
24262678.
83. Berman RM, Thase ME, Trivedi MH, Hazel JA, Marler SV, McQuade RD, et al. Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder. Neuropsychiatr Dis Treat. 2011; 7:303–312. PMID:
21655344.
84. Stewart JW, McGrath PJ, Fava M, Wisniewski SR, Zisook S, Cook I, et al. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int J Neuropsychopharmacol. 2010; 13:15–30. PMID:
19341509.
85. Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008; 165:342–351. PMID:
18172020.
86. Stewart JW. Treating depression with atypical features. J Clin Psychiatry. 2007; 68(Suppl 3):25–29. PMID:
17348764.
87. Liebowitz MR, Quitkin FM, Stewart JW, McGrath PJ, Harrison WM, Markowitz JS, et al. Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988; 45:129–137. PMID:
3276282.
88. Nelson JC, Thase ME, Bellocchio EE, Rollin LM, Eudicone JM, McQuade RD, et al. Efficacy of adjunctive aripiprazole in patients with major depressive disorder who showed minimal response to initial antidepressant therapy. Int Clin Psychopharmacol. 2012; 27:125–133. PMID:
22466058.
89. Nelson JC, Mankoski R, Baker RA, Carlson BX, Eudicone JM, Pikalov A, et al. Effects of aripiprazole adjunctive to standard antidepressant treatment on the core symptoms of depression: a post-hoc, pooled analysis of two large, placebo-controlled studies. J Affect Disord. 2010; 120:133–140. PMID:
19656577.
90. Trivedi MH, Thase ME, Fava M, Nelson CJ, Yang H, Qi Y, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008; 69:1928–1936. PMID:
19192475.
91. Thase ME, Demyttenaere K, Earley WR, Gustafsson U, Udd M, Eriksson H. Extended release quetiapine fumarate in major depressive disorder: analysis in patients with anxious depression. Depress Anxiety. 2012; 29:574–586. PMID:
22753280.
92. Bauer M, El-Khalili N, Datto C, Szamosi J, Eriksson H. A pooled analysis of two randomised, placebo-controlled studies of extended release quetiapine fumarate adjunctive to antidepressant therapy in patients with major depressive disorder. J Affect Disord. 2010; 127:19–30. PMID:
20884063.
93. Dunner DL, Laubmeier KK, Manos G, Forbes RA, Baker RA, Berman RM. Beneficial effects of adjunctive aripiprazole in major depressive disorder are not dependent on antidepressant therapy history: a post hoc analysis of 3 randomized, double-blind, placebo-controlled trials. Prim Care Companion CNS Disord. 2012; 14:DOI:
10.4088/PCC.12m01380. [Epub ahead of print].
94. Stewart TD, Hatch A, Largay K, Sheehan JJ, Marler SV, Berman RM, et al. Effect of symptom severity on efficacy and safety of aripiprazole adjunctive to antidepressant monotherapy in major depressive disorder: a pooled analysis. J Affect Disord. 2014; 162:20–25. PMID:
24766999.
95. Dunner DL, Laubmeier KK, Marler SV, Forbes RA, Baker RA. Efficacy of adjunctive aripiprazole in major depressive disorder: a pooled response quartile analysis and the predictive value of week 2 early response. Prim Care Companion CNS Disord. 2012; 14:DOI:
10.4088/PCC.11m01251. [Epub ahead of print].
96. Nelson JC, Rahman Z, Laubmeier KK, Eudicone JM, McQuade RD, Berman RM, et al. Efficacy of adjunctive aripiprazole in patients with major depressive disorder whose symptoms worsened with antidepressant monotherapy. CNS Spectr. 2014; 19:528–534. PMID:
24642260.
97. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009; 166:980–991. PMID:
19687129.
98. Shen H, He MM, Liu H, Wrighton SA, Wang L, Guo B, et al. Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos. 2007; 35:1292–1300. PMID:
17470523.
99. Chong SA, Tan CH, Khoo YM, Lee HS, Wong KE, Ngui F, et al. Clinical evaluation and plasma clozapine concentrations in Chinese patients with schizophrenia. Ther Drug Monit. 1997; 19:219–223. PMID:
9108654.
100. Ji L, Pan S, Marti-Jaun J, Hänseler E, Rentsch K, Hersberger M. Single-step assays to analyze CYP2D6 gene polymorphisms in Asians: allele frequencies and a novel *14B allele in mainland Chinese. Clin Chem. 2002; 48:983–988. PMID:
12089164.
101. Chen SJ, Hsiao YL, Shen TW, Chen ST. The effectiveness and safety of adjunctive aripiprazole in Taiwanese patients with antidepressant-refractory major depressive disorder: a prospective, open-label trial. J Clin Psychopharmacol. 2012; 32:56–60. PMID:
22198444.
102. Yoshimura R, Kishi T, Hori H, Ikenouchi-Sugita A, Katsuki A, Umene-Nakano W, et al. Comparison of the efficacy between paroxetine and sertraline augmented with aripiprazole in patients with refractory major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39:355–357. PMID:
22813840.
103. Nierenberg AA, Trivedi MH, Gaynes BN, Mitchell J, Davis LL, Husain MM, et al. Effectiveness study of Venlafaxine-XR combined with aripiprazole for chronic or recurrent major depressive disorder. Aust N Z J Psychiatry. 2009; 43:956–967.
104. Pae CU, Jeon HJ, Lee BC, Seo HJ, Kim SG, Park EJ, et al. Aripiprazole augmentation for treatment of patients with chronic or recurrent major depressive disorder: a 12-week prospective open-label multicentre study. Int Clin Psychopharmacol. 2013; 28:322–329. PMID:
23873293.
105. Jing Y, Kalsekar I, Curkendall SM, Carls GS, Bagalman E, Forbes RA, et al. Intent-to-treat analysis of health care expenditures of patients treated with atypical antipsychotics as adjunctive therapy in depression. Clin Ther. 2011; 33:1246–1257. PMID:
21840058.
106. Steffens DC, Nelson JC, Eudicone JM, Andersson C, Yang H, Tran QV, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011; 26:564–572. PMID:
20827794.
107. Jang H, Kim SH, Park SH, Choo IH, Kim SG. Psychiatric symptoms in temporal lobe epilepsy with left mesial hippocampal sclerosis. Psychiatry Investig. 2015; 12:274–277.
108. Geoffroy PA, Goddefroy G, Rolland B, Cottencin O. Efficacy of aripiprazole in comorbid addiction in bipolar disorder. CNS Neurosci Ther. 2012; 18:359–360. PMID:
22486848.
109. Jayaram N, Rao NP, Venkatasubramanian G, Behere RV, Varambally S, Gangadhar BN. Successful use of aripiprazole for delusional disorder with comorbid pituitary microadenoma: a case report. Psychosomatics. 2011; 52:395–397. PMID:
21777728.
110. Starrenburg FC, Bogers JP. How can antipsychotics cause Diabetes Mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur Psychiatry. 2009; 24:164–170. PMID:
19285836.
111. Koh JS, Ko HJ, Wang SM, Cho KJ, Kim JC, Lee SJ, et al. The relationship between depression, anxiety, somatization, personality and symptoms of lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Psychiatry Investig. 2015; 12:268–273.
112. Park SC, Lee HY, Sakong JK, Jun TY, Lee MS, Kim JM, et al. Distinctive clinical correlates of psychotic major depression: the CRESCEND study. Psychiatry Investig. 2014; 11:281–289.
113. Zetin M, Hoepner CT, Bjornson L. Rational antidepressant selection: applying evidence-based medicine to complex real-world patients. Psychopharmacol Bull. 2006; 39:38–104. PMID:
17065974.
114. Liu YM, Ou HT, Yang YK. Choice of generic versus brand-name antidepressants in a regulated prescription drug market: evidence from Taiwan. J Ment Health Policy Econ. 2014; 17:163–172. PMID:
25599280.
115. Donohue JM, Zhang Y, Aiju M, Perera S, Lave JR, Hanlon JT, et al. Impact of Medicare Part D on antidepressant treatment, medication choice, and adherence among older adults with depression. Am J Geriatr Psychiatry. 2011; 19:989–997. PMID:
22123272.
116. Owenby RK, Brown LT, Brown JN. Use of risperidone as augmentation treatment for major depressive disorder. Ann Pharmacother. 2011; 45:95–100. PMID:
21189367.
117. Dunner DL, Amsterdam JD, Shelton RC, Loebel A, Romano SJ. Efficacy and tolerability of adjunctive ziprasidone in treatment-resistant depression: a randomized, open-label, pilot study. J Clin Psychiatry. 2007; 68:1071–1077. PMID:
17685744.
118. Rocca P, Marchiaro L, Rasetti R, Rivoira E, Bogetto F. A comparison of paroxetine versus paroxetine plus amisulpride in the treatment of dysthymic disorder: efficacy and psychosocial outcomes. Psychiatry Res. 2002; 112:145–152. PMID:
12429360.
119. Rocca P, Fonzo V, Ravizza L, Rocca G, Scotta M, Zanalda E, et al. A comparison of paroxetine and amisulpride in the treatment of dysthymic disorder. J Affect Disord. 2002; 70:313–317. PMID:
12128243.
120. Zanardi R, Smeraldi E. A double-blind, randomised, controlled clinical trial of acetyl-L-carnitine vs. amisulpride in the treatment of dysthymia. Eur Neuropsychopharmacol. 2006; 16:281–287. PMID:
16316746.
121. Liebowitz M, Lam RW, Lepola U, Datto C, Sweitzer D, Eriksson H. Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: a randomized, placebo-controlled trial. Depress Anxiety. 2010; 27:964–976. PMID:
20734365.
122. Papakostas GI, Fava M, Baer L, Swee MB, Jaeger A, Bobo WV, et al. Ziprasidone augmentation of escitalopram for major depressivedisorder: efficacy results from a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2015; appiajp201514101251. [Epub ahead of print]. PMID:
26085041.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download