Abstract
This retrospective study was performed to analyze the long-term outcome of topical corticosteroid treatment for severe dry eye associated with Sjögren's syndrome (SS). Patients who had severe dry eye associated with SS were topically treated with loteprednol etabonate 0.5% (group A, n=66) or fluorometholone 0.1% (group B, n=67) twice daily and were followed up for 2 years. Visual acuity (VA), intraocular pressure (IOP), Schirmer test, tear film breakup time (BUT), keratoepitheliopathy, and symptom scores were measured at baseline and 6, 12, 18, and 24 months after treatment. VA and IOP were not changed significantly during follow-up in either group. Schirmer test results, keratoepitheliopathy, and symptom scores at 6, 12, 18, and 24 months (p<0.05) and tear film BUT at 12, 18, and 24 months (p<0.05) significantly improved after treatment compared with baseline in both groups. No significant differences between the groups were found in any parameter during follow-up. At 24 months, the number of patients with IOP elevation of more than 2 mmHg compared with baseline was 4 in group A (6.1%) and 9 in group B (13.4%). The mean IOP in these patients was lower in group A than in group B (15.00±0.82 mmHg versus 16.50±1.12 mmHg; p=0.04). Long-term application of low-dose topical corticosteroids is effective for controlling signs and symptoms of chronic, severe dry eye associated with SS. Loteprednol etabonate 0.5% may have a lower risk for IOP elevation than fluorometholone 0.1%.
Dry eye is one of the most common problems encountered by ophthalmologists. About 14% to 33% of the worldwide population suffers from dry eye.1 Dry eye disease is defined as "a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface, accompanied by increased osmolarity of the tear film and inflammation of the ocular surface."2 Ocular surface inflammation is known to play a key role in the pathogenesis of dry eye disease. Therefore, topical anti-inflammatory medications such as corticosteroids, cyclosporine A, and nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to artificial tears have been used in patients with dry eye disease.
Corticosteroids induce several anti-inflammatory effects.3 For instance, they suppress inflammatory cytokines and chemokines, decrease the expression of cell adhesion molecules, and stimulate apoptosis of lymphocytes. Topical corticosteroids are useful in a variety of inflammatory ocular surface diseases, including dry eye and allergic conjunctivitis. However, adverse events have been reported with their use, such as increased intraocular pressure (IOP), development of cataract, increased risk of infection, and delayed wound healing.4 On the other hand, topical cyclosporine A has been shown to suppress activated T cells and proinflammatory cytokines in the conjunctiva and to increase the numbers of goblet cells and the proliferative activity of the epithelium, leading to an increase in tear production.5,6,7 However, cyclosporine A may need to be used for several months before any therapeutic effect is observed, and some patients may discontinue medications owing to ocular discomforts associated with either the pH of the emulsion or a direct toxic effect of the cyclosporine.8
Sjögren's syndrome (SS) is a systemic autoimmune disease, extending from sicca symptoms, such as xerophthalmia and xerostomia, to systemic manifestations (extraglandular involvement).9 Dry eye with SS is representative of aqueous-deficient dry eye associated with lacrimal gland inflammation. Patients with SS have more severe clinical features and chronic inflammation than do patients without SS.10,11 Therefore, anti-inflammatory medications are often needed in these patients.
To date, several studies have been conducted of the effectiveness of anti-inflammatory treatment for dry eye associated with SS. In those studies, short-term improvements were observed in subjective and objective parameters of dry eye after the use of topical corticosteroids, cyclosporine A, or NSAIDs.12,13,14,15 Although topical cyclosporine A is commonly used for the long-term treatment of dry eye disease, clinicians frequently encounter patients with SS-dry eye who do not improve with treatment. Currently, no studies involving the long-term outcome of treatment with topical corticosteroids in dry eye associated with SS have been reported. Accordingly, the purpose of this study was to analyze the long-term efficacy and safety of topical low-dose corticosteroid treatment for chronic dry eye associated with SS in patients with severe inflammation with central corneal staining whose symptoms were refractory to topical cyclosporine A or artificial tears.
In this retrospective, clinical comparative analysis, participants who met the eligibility criteria were enrolled from a clinical database of Korean patients who were treated for severe SS-dry eye between January 2009 and September 2014 at the Department of Ophthalmology, Chonnam National University Medical School and Hospital. The study was conducted in accordance with the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained from the Institutional Review Board of the Chonnam National University Hospital.
Dry eye was diagnosed according to the patients' report of ocular discomfort (soreness, scratchiness, grittiness, dryness, and/or burning sensation), tear film breakup time (BUT), Schirmer test, and corneal staining with fluorescein as well as other general ophthalmic examinations. To be included in the study, all patients had to meet the following criteria: chronic dry eye disease with International Dry Eye Workshop (DEWS) level III (marked, central corneal staining and Schirmer test results <5 mm/5 minutes or tear film BUT <5 s) or higher, Sjögren's syndrome (primary or secondary) according to the criteria proposed by the American-European Consensus Group,16 and 18 years of age or older. Patients who did not respond to topical cyclosporine A and artificial tears and who were treated with topical corticosteroids and sodium hyaluronate 0.1% artificial tears for at least 24 months were enrolled.
Exclusion criteria included active ocular infection, inflammation not associated with dry eye, recent use of contact lenses (within 1 month), punctal plug insertion, history of ocular surgery (within the past 3 months), use of systemic medication potentially interfering with tear production or evaporation (such as dopaminergics, benzodiazepines), history of glaucoma or suspicion of glaucoma, or history of systemic diseases such as thyroid disease, diabetes mellitus, hypertension, peptic ulcer, and osteoporosis. When both eyes satisfied the inclusion criteria, the right eye from each patient was selected for analysis in the study.
Of a total of 612 patients with SS-dry eye, this study included 133 eyes of 133 patients. Group A consisted of 66 patients treated with topical loteprednol etabonate 0.5% (Lotemax, Bausch & Lomb, Rochester, NY, USA), in addition to sodium hyaluronate 0.1% artificial tears (Kynex, Alcon Korea, Seoul, Korea). Group B consisted of 67 patients treated with topical fluorometholone 0.1% (Ocumetholone, Samil Pharm. Co., Korea) in addition to sodium hyaluronate 0.1% artificial tears. Topical corticosteroids were applied as 1 drop twice daily, and artificial tears were applied as 1 drop six times per day. All patients were followed up bimonthly.
All patients underwent ophthalmic examinations including uncorrected visual acuity (UCVA), manifest refraction, IOP measurements with Goldmann applanation tonometry, slit-lamp biomicroscopy, fundus examination, Schirmer test, tear film BUT measurements, keratoepitheliopathy score assignment with fluorescein, and the grading of dry eye symptoms before treatment and after 6, 12, 18, and 24 months of treatment. UCVA measured with the Snellen chart was expressed as logMAR. Considering diurnal variation in IOP, an IOP elevation of more than 2 mmHg compared to baseline was regarded as a significant IOP elevation in this study.17
The tear film and ocular surface parameters were measured as follows: Schirmer test, tear film BUT, and keratoepitheliopathy score. All tear film and ocular surface parameters were measured by the same investigator (Y.K.C.). Schirmer test was performed without anesthetic, measuring the total tear secretion (basic and reflex) in each eye. The eyes were gently dried with tissue paper over closed lids to mop up any excess secretion. The standard strip was folded 5 mm from one end and inserted at the junction of the middle and outer third of the lower lids. After 5 minutes, the filter papers were removed. The length of the wet strip was measured by using a millimeter scale, and the average of 2 measurements for each eye was calculated.18 Tear film BUT was evaluated 2 minutes after instillation of 2µl of 0.5% fluorescein. The subjects were asked to blink several times and then to keep their eyes open. The time in seconds between the last complete blink and the appearance of the first corneal black spot was measured and the mean value of the measurements was recorded.19 Keratoepitheliopathy was scored by multiplying the area score (0-3) by the density score (0-3) after staining with 1% unpreserved fluorescein. The staining area was graded on a numerical scale of 0 to 3, with 0 representing no punctate staining, 1 representing less than one-third, 2 representing one-third to two-thirds, and 3 representing more than two-thirds staining. The staining density was graded according to the number of punctate epithelial erosions (PEEs) as follows: 0, no PEE; 1, less than 5; 2, 5-30; 3, more than 30 PEEs.20 Subjective dry eye symptoms such as burning, foreign body sensation, and dryness were graded on a numerical score of 0 to 4, with 0 representing no symptoms, 1 representing mild symptoms that did not constitute discomfort, 2 representing moderate symptoms causing discomfort for less than half a day, 3 representing severe symptoms causing discomfort for at least half a day, and 4 representing very severe symptoms causing discomfort and interfering with normal activities.20 Clinical signs and symptoms were recorded by using standard clinical medical records over a period of at least 24 months after treatment.
The Statistical Package for the Social Sciences (SPSS, version 17.0, Chicago, IL, USA) was used for statistical analysis. The normality of distribution was verified by using the Shapiro-Wilk normality test. Results are presented as mean±standard deviation. Patients' demographic data were analyzed by using descriptive statistics. Differences in various parameters between groups were evaluated by using the chi-square test and the independent t-test or Mann-Whitney U test. To compare changes in parameters in both groups, repeated-measures analysis of variance (ANOVA) was used. A post hoc test with Bonferroni adjustment was then used to determine significance between pairs of relevant groups. The probability level of p<0.05 was considered to be statistically significant.
Table 1 summarizes the demographics and baseline characteristics of the patients. In group A, the mean age of the 2 men and 64 women was 53.17±11.82 years. In group B, the mean age of the 3 men and 64 women was 52.02±12.23 years. There were no statistically significant differences in age, sex, UCVA, refractive errors, or IOP between the groups. In addition, the tear film and ocular surface parameters showed no significant differences (p>0.05).
Group A included 48 patients (72.7%) with primary SS and 18 patients (27.3%) with secondary SS, including 10 with rheumatoid arthritis, 6 with systemic lupus erythematosus, and 2 with systemic sclerosis. In group B, 50 patients (74.6%) had primary SS and 17 patients (25.4%) had secondary SS, including 11 with rheumatoid arthritis, 4 with systemic lupus erythematosus, and 2 with ankylosing spondylitis. All patients were treated with oral hydroxychloroquine by rheumatologists. The patients with primary SS were additionally treated with oral pilocarpine.
Schirmer test results at baseline and 6, 12, 18, and 24 months after treatment are shown in Fig. 1A. The mean scores in group A were 4.38±1.04 mm at baseline, 4.88±1.15 mm at 6 months, 5.50±1.20 mm at 12 months, 5.88±1.10 mm at 18 months, and 5.90±1.08 mm at 24 months after treatment, and respective scores in group B were 4.36±1.50 mm at baseline, 4.80±1.35 mm at 6 months, 5.20±1.24 mm at 12 months, 5.35±1.43 mm at 18 months, and 5.48±1.30 mm at 24 months. The mean Schirmer's scores at all follow-up periods were significantly increased compared with baseline in both groups (all p<0.05).
Fig. 1B illustrates tear film BUTs at baseline and 6, 12, 18, and 24 months after treatment. The mean tear film BUTs in group A were 3.66±1.00 s, 3.74±0.95 s, 3.84±1.05 s, 4.00±1.05 s, and 4.35±1.19 s at baseline, 6, 12, 18, and 24 months, respectively. The respective values in group B were 3.57±0.97 s, 3.75±1.10 s, 3.90±1.10 s, 4.20±1.24 s, and 4.21±1.29 s. In both groups, the mean tear film BUT at 12, 18, and 24 months after treatment showed a significant increase compared with baseline (all p<0.05).
The mean keratoepitheliopathy scores at baseline, 6, 12, 18, and 24 months were 5.75±1.19, 5.06±1.70, 4.76±1.30, 4.45±1.30, and 4.41±1.04 in group A, and 5.72±1.35, 5.10±1.25, 4.85±1.66, 4.70±1.83, and 4.45±1.40 in group B, respectively (Fig. 1C). The mean keratoepitheliopathy score in all follow-up periods showed a statistically significant decrease (all p<0.05) compared with baseline in both groups.
The mean symptom scores in group A were 3.04±0.85, 2.58±0.90, 2.45±0.94, 2.20±1.18, and 2.17±1.20 at baseline, 6, 12, 18, and 24 months, respectively, and the respective mean scores in group B were 2.99±0.89, 2.60±0.80, 2.40±0.99, 2.25±1.00, and 2.21±1.11 (Fig. 1D). Similar to the Schirmer test and keratoepitheliopathy score, significant improvements in the dry eye symptom were observed at all follow-up periods in both groups (all p<0.05). There were no significant differences in the Schirmer test results, tear film BUT, keratoepitheliopathy score, or symptom score between group A and group B at any time point after treatment.
The mean UCVA at 6 months was 0.38±0.12 in group A and 0.38±0.14 in group B. At 12, 18, and 24 months, the mean UCVA was, respectively, 0.38±0.15, 0.40±0.12, and 0.40±0.18 in group A and 0.40±0.13, 0.41±0.15, and 0.40±0.15 in group B. There were no significant differences at any follow-up visit compared with baseline in either group. In addition, no significant differences between groups A and B were found during follow-up.
The mean IOP values were 13.80±1.21 mmHg at baseline, 13.99±1.20 mmHg at 6 months, 14.00±1.80 mmHg at 12 months, 14.06±1.18 mmHg at 18 months, and 14.23±1.50 mmHg at 24 months in group A, and 13.74±1.20 mmHg, 14.12±1.20 mmHg, 14.40±1.21 mmHg, 14.34±1.32 mmHg, and 14.60±1.25 mmHg at baseline, 6, 12, 18, and 24 months, respectively, in group B (Fig. 2). No significant elevations in the mean IOP value were found in either group at any follow-up visit compared with baseline. In addition, there were also no significant differences between groups A and B. No patients with IOP elevation required anti-glaucoma medications.
IOP elevations of more than 2 mmHg compared with baseline were analyzed at 6, 12, 18, and 24 months after treatment in both groups: one patient at 6 months (1.5%), one at 12 months (1.5%), three at 18 months (4.5%), and four at 24 months (6.1%) in group A, and two patients at 6 months (3.0%), three at 12 months (4.5%), six at 18 months (9.0%), and nine at 24 months (13.4%) in group B (Fig. 3A). Fig. 3B illustrates the changes in the mean IOP value in patients with an IOP elevation of more than 2 mmHg at 24 months compared with baseline. A significant difference in the mean IOP value between the groups was found at 24 months after treatment (15.00±0.82 mmHg in group A versus 16.50±1.12 mmHg in group B; p=0.04).
According to the DEWS guideline, anti-inflammatory treatment in dry eye disease is recommended when the severity level reaches level II or higher.2 Dry eye in SS is caused by lacrimal hypo-secretion with inflammatory changes in the lacrimal gland, leading to the presence of inflammatory mediators in the tears. Several studies have reported that dry eye associated with SS is more severe than non-SS dry eye.10,11 Therefore, topical anti-inflammatory medications such as corticosteroids, NSAIDs, and cyclosporine A 0.05% are needed to treat SS-dry eye. The anti-inflammatory mechanism of cyclosporine A 0.05% is limited to the inhibition of T-cell activation, whereas corticosteroids have multifaceted effects on anti-inflammatory mechanisms as well as immunomodulation.21 Corticosteroids exert their biological effects through nuclear glucocorticoid receptors. Corticosteroids inhibit interleukin-2 (IL-2) production by T cells.22 Furthermore, corticosteroids down-regulate the production of the pro-inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) by monocytes and macrophages.23 Avunduk et al.24 noted that topical corticosteroids were superior for improving the clinical parameters of dry eye compared with NSAIDs in both patients with and patients without SS. Some case studies have been carried out with the short-term use of topical corticosteroids to treat SS-dry eye, showing improvements of signs and symptoms.12,14 Marsh et al.,12 in a retrospective, noncomparative case study, reported that application of topical nonpreserved methylprednisolone 3 to 4 times per day for 2 weeks was an effective treatment option for patients with SS-associated severe keratoconjunctivitis sicca, leading to relief of irritation symptoms and improvement of the corneal staining score. Aragona et al., in a prospective, placebo-controlled study, evaluated 40 patients with SS.14 The treatment group was treated with topical 0.1% clobetasone butyrate twice daily for 30 days. Corneal and conjunctival stain significantly improved at day 15, and the symptoms score improved at day 30. No changes in IOP or fundus examination were observed in the placebo group or the treatment groups at either time point.
In the present study, the Schirmer test results, tear film BUT, keratoepitheliopathy, and subjective symptoms improved during the follow-up period after treatment with both loteprednol etabonate 0.5% and fluorometholone 0.1%. Topical corticosteroids can decrease ocular surface inflammation and promote a healthy tear composition, leading to the recovery of epithelial damage. Interestingly, the tear film BUT did not significantly improve at 6 months after treatment. This may be because all patients in the present study had severe dry eye with marked corneal staining.
Generally, the long-term use of corticosteroids has been known to have a risk of adverse events, such as increased IOP, development of cataract, and infection. In the present study, patients with chronic, severe dry eye with SS, which was refractory to topical cyclosporine A or NSAIDs, were treated with long-term, low-frequency topical corticosteroids. No infectious lesions were observed during the follow-up periods in either group. UCVA and IOP at 6, 12, 18, and 24 months after corticosteroid treatment had no statistically significant changes compared with baseline in either the loteprednol etabonate or fluorometholone group. We suppose that several factors may influence the absence of significant changes in mean IOP values in our study. First, both corticosteroids have a lower tendency to induce IOP elevation compared with other corticosteroids such as dexamethasone or methylprednisolone. Second, we administrated topical corticosteroids at a low frequency. Third, we excluded patients with glaucoma or glaucoma suspect and known steroid responders.
Diurnal variations in IOP in the normal eye range between 2 and 10 mmHg.17 To minimize the diurnal variation in IOP, we made an effort to measure IOP at the same time of day during follow-up visits for each patient, and analysis was performed to identify the patients with an IOP elevation of more than 2 mmHg compared with baseline. The number of these patients at each follow-up visit differed between the groups. At 24 months after treatment, the proportions of patients were 6.9% in the loteprednol etabonate group and 15.3% in the fluorometholone group. The mean IOP value was significantly lower in the loteprednol etabonate group than in the fluorometholone group.
Loteprednol etabonate is a novel ester-based corticosteroid, the 17β-chloromethyl ester of Δ1-cortienic acid etabonate, a derivative of the prednisolone metabolite, Δ1-cortienic acid.25 Loteprednol etabonate is highly lipophilic and has strong binding to the glucocorticoid receptor. Also, it is rapidly metabolized after action by tissue esterases, thereby limiting any potential adverse effects.26 A number of studies have demonstrated that loteprednol etabonate has potent anti-inflammatory efficacy with less impact on IOP elevation than other corticosteroids.27 Consistent with previous reports, in the present study, the risk of IOP elevation was lower with the use of loteprednol etabonate than with fluorometholone.
Although some patients experienced IOP elevation, no elevation to values higher than 21 mmHg was observed in either group. In addition, no abnormalities of the optic nerve head, retinal nerve fiber layer, or visual field were found in the patients with IOP elevation. Nevertheless, it is important to recognize that there is a possibility of IOP elevation, which could lead to glaucomatous optic nerve damage.
Our study had several limitations. The study design was retrospective, and the sample size was relatively small. A further prospective, randomized, large-scale clinical trial could confirm and better address our results. Second, this study had no control group. We included patients who had chronic dry eye disease with DEWS level III or higher. Inflammation of the ocular surface such as severe central corneal staining was not controlled with lubricants such as topical cyclosporine A in all patients. Therefore, in this retrospective study, patients who were treated with lubricants only could not be recruited. Third, although we performed slit lamp biomicroscopy on every patient over the course of the study, cataract grading by the Lens Opacity Classification System (LOCS) was not evaluated. However, there was no remarkable development or progression of cataract in the patients of this study. In addition, no significant differences in UCVA or visual symptoms associated with cataract such as clouded vision, glare, and sensitivity to light were found during the follow-up period compared with baseline. Further studies on assessment of cataract grading are warranted. Finally, although effort was made to measure IOP at the same time point during the follow-up visits for each patient, it is possible that IOP spikes in between the follow-up visits were missed.
In conclusion, long-term application of low-dose topical corticosteroids, such as loteprednol etabonate 0.5% or fluorometholone 0.1%, can be effective to control signs and symptoms of chronic, severe dry eye associated with SS. Loteprednol etabonate 0.5% may have a lower risk for IOP elevation than fluorometholone 0.1%. Although long-term use of topical corticosteroids seems to be an effective treatment option, it is important to monitor possible side effects, including IOP elevation.
References
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136:318–326. PMID: 12888056.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92. PMID: 17508116.
3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–1723. PMID: 16236742.
4. Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000; 11:478–483. PMID: 11141645.
5. Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986; 31:159–169. PMID: 3544293.
6. Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991; 35:369–385. PMID: 2038720.
7. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005; 89:1363–1367. PMID: 16170133.
8. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–639. PMID: 10768324.
9. Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren's syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012; 96:1498–1503. PMID: 23001257.
10. Goto E, Matsumoto Y, Kamoi M, Endo K, Ishida R, Dogru M, et al. Tear evaporation rates in Sjögren syndrome and non-Sjögren dry eye patients. Am J Ophthalmol. 2007; 144:81–85. PMID: 17509507.
11. Horwath-Winter J, Berghold A, Schmut O, Floegel I, Solhdju V, Bodner E, et al. Evaluation of the clinical course of dry eye syndrome. Arch Ophthalmol. 2003; 121:1364–1368. PMID: 14557170.
12. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999; 106:811–816. PMID: 10201607.
13. Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjögren's syndrome. Acta Ophthalmol (Copenh). 1994; 72:438–442. PMID: 7825408.
14. Aragona P, Spinella R, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren syndrome. Eur J Ophthalmol. 2013; 23:368–376. PMID: 23225089.
15. Aragona P, Stilo A, Ferreri F, Mobrici M. Effects of the topical treatment with NSAIDs on corneal sensitivity and ocular surface of Sjögren's syndrome patients. Eye (Lond). 2005; 19:535–539. PMID: 15184937.
16. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. Sjögren's International Collaborative Clinical Alliance (SICCA) Research Groups. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64:475–487. PMID: 22563590.
17. de Venecia G, Davis MD. Diurnal variation of intraocular pressure in the normal eye. Arch Ophthalmol. 1963; 69:752–757. PMID: 14026102.
18. Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007; 144:86–92. PMID: 17493572.
19. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001; 108:586–592. PMID: 11237914.
20. Kaido M, Goto E, Dogru M, Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900. PMID: 15121365.
21. Samudre SS, Lattanzio FA Jr, Williams PB, Sheppard JD Jr. Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004; 20:533–547. PMID: 15684812.
22. Daynes RA, Araneo BA. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989; 19:2319–2325. PMID: 2606141.
23. Linden M, Brattsand R. Effects of a corticosteroid, budesonide, on alveolar macrophage and blood monocyte secretion of cytokines: differential sensitivity of GM-CSF, IL-1 beta, and IL-6. Pulm Pharmacol. 1994; 7:43–47. PMID: 8003851.
24. Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003; 136:593–602. PMID: 14516798.
25. Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000; 20:58–101. PMID: 10608921.
26. Druzgala P, Hochhaus G, Bodor N. Soft drugs--10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991; 38:149–154. PMID: 2004037.
27. Loteprednol Etabonate US Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am J Ophthalmol. 1999; 127:537–544. PMID: 10334346.
FIG. 1
Changes of Schirmer test (A), Tear film break-up time (BUT), (B) Keratoepitheliopathy, (C) and symptom score (D) in the loteprednol etabonate 0.5% and fluorometholone 0.1% groups. Group A: loteprednol etabonate group, Group B: fluorometholone group, Tear film-BUT: tear film break-up time, *p<0.05 compared to the baseline.
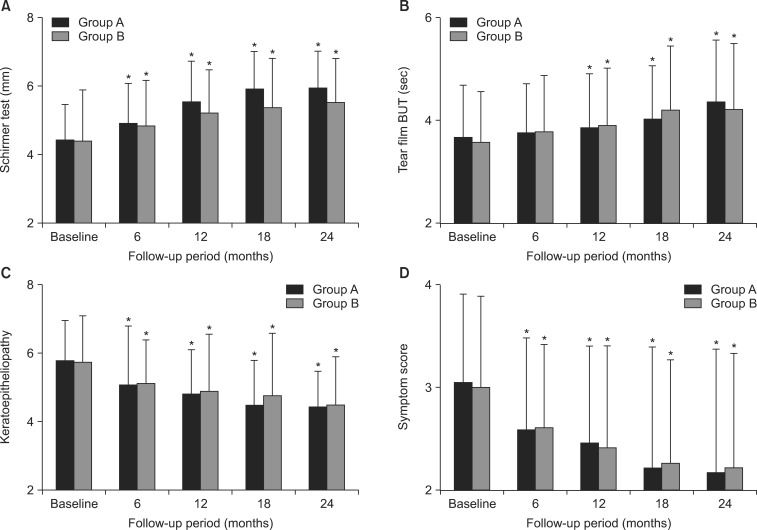
FIG. 2
Changes of intraocular pressure (IOP) in the loteprednol etabonate 0.5% and fluorometholone 0.1% groups. IOP: intraocular pressure, Group A: loteprednol etabonate group, Group B: fluorometholone group.
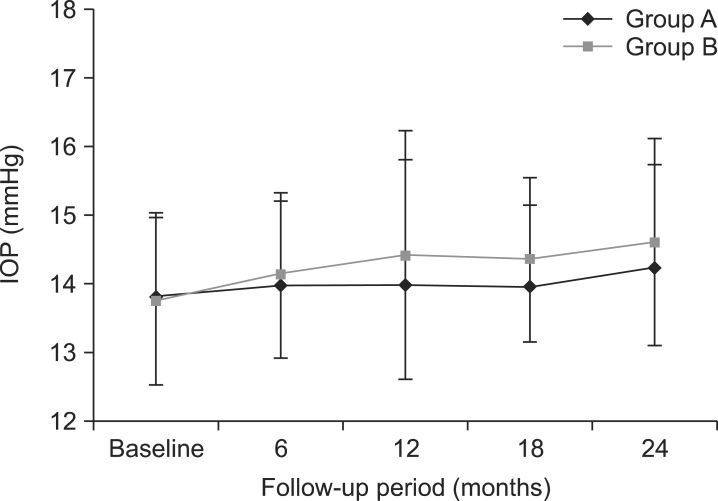
FIG. 3
(A) Proportion of patients with intraocular pressure (IOP) elevation by more than 2 mmHg compared to the baseline at 6, 12, 18 and 24 months in the loteprednol etabonate 0.5% and fluorometholone 0.1% groups. (B) Mean IOP value in patients with IOP elevation by more than 2 mmHg compared to the baseline at 24 months. IOP: intraocular pressure, Group A: loteprednol etabonate group, Group B: fluorometholone group, *p values (comparison between group A and group B)<0.05.
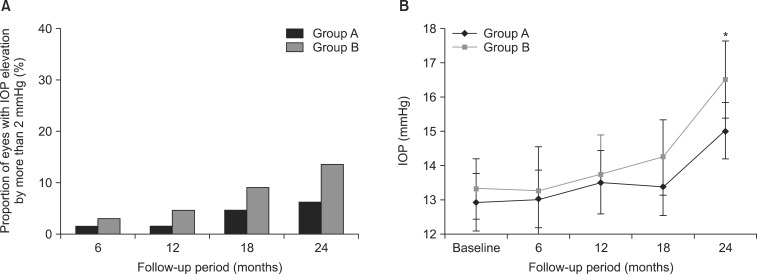
TABLE 1
Baseline characteristics of patients who were treated with topical corticosteroids and sodium hyaluronate (SH) 0.1% artificial tears for severe dry eye associated with Sjögren's syndrome
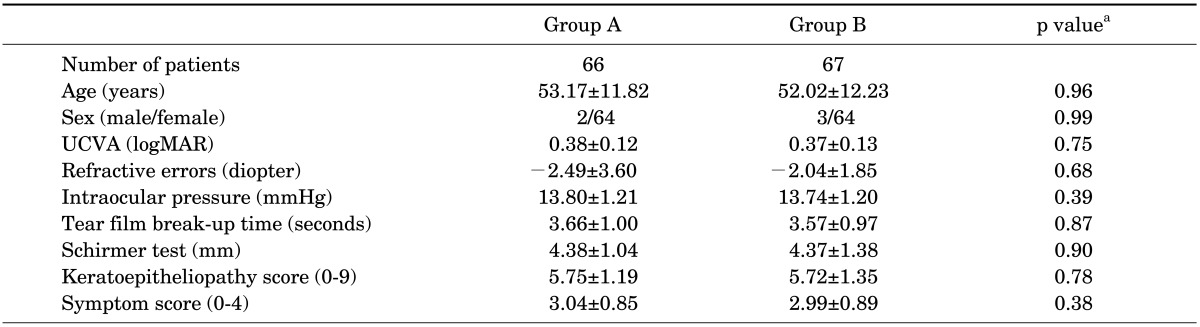
UCVA: Uncorrected visual acuity, logMAR: log of the minimum angle of resolution, Group A: Loteprednol etabonate 0.5% group, Group B: Fluorometholone 0.1% group. Data are expressed as the mean±standard deviation. aChi-square test (sex distribution) and independent t-test or Mann-Whitney U test (other variables).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download