Abstract
Idiopathic membranous glomerulonephritis (IMGN) is commonly diagnosed in adults with proteinuria. Rapid deterioration of renal function is a rare complication of IMGN, except when accompanied by renal vein thrombosis, malignant hypertension, or other underlying disease, including lupus nephritis. Here, we present a case of rapid deterioration of renal function in a patient with MGN superimposed with anti-neutrophil cytoplasmic antibody (ANCA)-associated rapidly progressive crescentic glomerulonephritis (RPGN). Overall, about 20 cases of MGN with ANCA-associated RPGN have been reported. This case of biopsy-proven MGN with ANCA-associated RPGN is the first to be reported in Korea.
Idiopathic membranous glomerulonephritis (IMGN) is commonly diagnosed in adults with proteinuria.1 Renal function is preserved in most cases. Rapid deterioration in renal function is a rare complication of IMGN, except when accompanied by renal vein thrombosis, malignant hypertension, or other underlying disease, including lupus nephritis.2 A few cases of MGN with anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis (GN) at presentation or crescentic transformation from previous MGN have been reported very rarely.3 All cases of crescentic transformation from MGN were diagnosed within the past 13 months, and MGN with anti-glomerular basement membrane (GBM) antibodies was only recently reported in Korea.45 However, no cases of MGN with superimposed ANCA-associated GN have been reported in Korea.
Here, we present a case of rapid deterioration of renal function in a patient with MGN superimposed with ANCA-associated rapidly progressive crescentic GN (RPGN).
A 65-year-old male was admitted to the hospital for further evaluation of a rapid increase in serum blood urea nitrogen (BUN) and creatinine. He had been given a diagnosis of hypertension 9 months earlier and was being treated with 5 mg amlodipine twice daily and 12.5 mg of carvedilol. He had no medical history except hypertension. At the time of the hypertension diagnosis, a complete blood count showed hemoglobin, 13.8 g/dL; BUN, 18.1 mg/dL; and serum creatinine, 1.1 mg/dL. The patient's serum creatinine level 150 days before admission was 1.2 mg/dL, and urinalysis showed protein 2+. The patient's renal function had declined 126 days earlier, and his serum creatinine had increased to 2.7 mg/dL. The primary physician recommended an evaluation of the deteriorated renal function but the patient refused.
The patient complained of frequency and nocturia beginning 30 days earlier and chest discomfort beginning 15 days earlier. He was admitted to another hospital before being admitted to our hospital. At that time, his laboratory results were as follows: hemoglobin, 12.2 g/dL; serum BUN, 37.6 mg/dL; and creatinine, 2.9 mg/dL. A urine dipstick test gave the following results: protein 2+ and erythrocytes 2+. The patient was transferred to our hospital for further evaluation.
On admission, a physical examination revealed 2+ pretibial pitting edema. In addition, the patient's body weight had increased from 73 to 76 kg in the past 3 months. He was pale and appeared unwell. His blood pressure was 170/84 mm Hg, his heart rate was 72/min, his respiratory rate was 20/min, and his body temperature was 36.6℃. The initial laboratory findings were as follows: white blood cell (WBC) count, 9,770/µL; hemoglobin, 7.4 g/dL; platelets, 188,000/µL; serum total protein, 6.2 g/dL; albumin, 3.1 g/dL; total cholesterol, 131 mg/dL; total calcium, 7.7 mg/dL; and phosphorus, 7.2 mg/dL. The patient's BUN and creatinine levels had increased to 93 and 9.7 mg/dL, respectively. A urine dipstick analysis showed protein 3+ and erythrocytes 3+. The urine protein to creatinine ratio was 5.62. Serological tests were negative for anti-nuclear antibody, immunoglobulins (IgG, IgA, and IgM), complement (C3 and C4), and protein electrophoresis. However, the patient was positive for p-ANCA (1:8 antibody index, myeloperoxidase [MPO]).
We evaluated the patient for hidden malignancy by use of duodenoscopy, colonoscopy, and chest computed tomography (CT) for a secondary MGN evaluation. However, we found no evidence of cancer, except for a gastric ulcer scar and a colonic adenoma. Moreover, no evidence of viral hepatitis was revealed.
Both chest X-ray and a subsequent chest CT scan showed cardiomegaly, traction bronchiectasis, and a honeycomb pattern in both lungs. A paranasal sinus series revealed increased opacity in the left maxillary sinus.
Ultrasound scanning of the kidneys showed a normal size (right: 11.18 cm; left: 11.74 cm) and nearly normal echogenic kidneys.
The renal cortex and medulla biopsies both consisted of 19 glomeruli: 8 (42.1%) showed global sclerosis and 9 tufts (47.4%) were circumferentially or segmentally fibroepithelial or fibrous crescents (epithelial, 2; fibroepithelial, 6; and fibrous, 1) (Fig. 1). The remaining tufts showed diffuse thickening of capillary walls with focal increases with a mononuclear cell infiltrate.
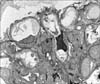
Direct immunofluorescence (IF) tests showed IgG, IgA, and C3 granular deposits along the capillary walls (Fig. 2).
An electron microscopic analysis revealed that the glomerular capillary walls had epimembranous and intramembranous electron-dense deposits. The overlying epithelial foot processes were diffusely effaced with microvillous transformation (Fig. 3). The findings were stage II-III/IV membranous GN with crescentic GN.
Chest X-rays and CT scans showed traction bronchiectasis, honeycombing in both lungs, and a ground- glass opacity in the right middle lobe and posterior basal segment of the right lower lobe. A pulmonologist said that this sign was unlikely to be related to ANCA-associated lung involvement or pulmonary hemorrhage. Because the patient's initial serum creatinine level was >5.8 mg/dL, we recommended plasmapheresis. However, he refused. Instead, therapy consisted of 500 mg of intravenous methylprednisolone pulse therapy for 3 days, followed by 60 mg/day of prednisolone and 100 mg/day of cyclophosphamide.
Despite this immunosuppressive treatment, the patient's serum creatinine level increased from 9.7 to 12.1 mg/dL and his total CO2 level decreased from 20 to 15.8 mEq/L on hospital day 7. In addition, his body weight increased by 4 kg. Acute hemodialysis was provided without recovery of renal function.
Regular hemodialysis was planned for 3 times per week, and the immunosuppressive therapy with prednisolone and cyclosporine was continued at the time of discharge on hospital day 9.
The patient's kidneys did not respond, despite the prednisolone and cyclosporine therapy during outpatient follow-up, and the immunosuppressive drugs were tapered.
"Subepithelial deposits of immunoglobulins and complement" are a characteristic feature of MGN. In addition, ANCA-associated vasculitis is one of the main vasculitis syndromes in which ANCA circulates against proteinase-3 and MPO. The clinical course is rapidly progressive GN; crescentic GN with extracapillary proliferation is observed histologically, few or no immunoglobulin deposits are seen on electron microscopy, and mild or absent IF staining for immunoglobulins or complement is seen.6
Because of the different pathophysiologies between IMGN and crescentic vasculitis, crescent formation is rarely seen in patients with IMGN. However, when crescents are detected in a patient with MGN, the possibility of mixed International Society of Nephrology/Renal Pathology Society class V lupus nephritis and class III or IV disease should be considered. As a second possible diagnosis, concurrent anti-GBM disease could be considered without clinical and serological evidence for lupus. The coexistence of MGN and ANCA-associated vasculitis is a third possible diagnosis but is exceptionally rare.7
MGN can be primary or secondary. Patients with primary MGN typically only have subepithelial deposits of immunoglobulins and complement.8 However, subendothelial and mesangial deposits in addition to subepithelial deposits are common in patients with secondary MGN. Only epimembranous and intramembranous deposits were found in our patient, and the mesangial space was not involved with the deposits. It is likely that this patient had primary MGN rather than secondary MGN.
Because MGN with ANCA-associated GN is rare, the standard treatment approach is not well described. Most nephrologists use cyclophosphamide and corticosteroid-based treatments according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines for pauci-immune focal and segmental necrotizing GN. The KDIGO guidelines also recommend adjunctive plasmapheresis therapy for this special patient population. Jayne et al. investigated the effect of plasmapheresis in a large, multicenter controlled trial including 137 patients with ANCA vasculitis confirmed by kidney biopsy and whose serum creatinine was >500 µmol/L (5.8 mg/dL).9 The patients randomly received either plasmapheresis or 300 mg of intravenous methylprednisolone. The risk reduction for progression to end-stage renal disease was 24% in the plasmapheresis group at 12 months, although the patient survival and severe adverse event rates were not significantly different between the groups.
The terms ANCA-associated crescentic GN and pauci-immune GN are typically used synonymously. However, Haas and Eustace examined 126 cases of ANCA-associated GN and found that 54% of ANCA-associated GN cases showed glomerular immune complex deposits and 87% showed positive IF findings, although the staining was relatively weak.6 Additionally, these cases showed a higher median level of proteinuria and a higher median percentage of glomeruli with crescents. Based on this result and the results of a cohort study, the prognosis of patients with MGN with ANCA-associated GN is likely to be worse than that of patients with ANCA-associated GN alone.10
In conclusion, we have reported the first case of MGN with ANCA-associated GN that presented coincidentally. Our suggestion is that if clinical ANCA-associated GN shows immune deposits in kidney biopsy or nephriticrange proteinuria or if crescents are found in a kidney biopsy of a patient with clinical MGN, dual glomerulopathy should be considered. Both cases would be expected to have poorer prognosis than a case of GN alone. In a therapeutic aspect, if a kidney biopsy shows electron-dense deposits with glomerular crescents, immunosuppressive therapy and plasmapheresis should be considered to slow the progression to end-stage renal disease.
Figures and Tables
FIG. 1
The glomeruli were segmentally involved with epithelial crescentic proliferation. The remaining glomerular capillaries appeared thickened (Periodic acid-Schiff stain, ×400).
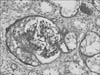
FIG. 2
(A) Direct immunofluorescence (IF) revealed granular IgG deposits along the capillary walls (anti-human IgG, ×200). (B) Direct IF revealed granular IgA deposits along the capillary walls (anti-human IgA, ×200). (C) Direct IF revealed granular C3 deposits along the capillary walls (anti-human C3, ×200).

References
1. Ponticelli C. Membranous nephropathy. J Nephrol. 2007; 20:268–287.
2. James SH, Lien YH, Ruffenach SJ, Wilcox GE. Acute renal failure in membranous glomerulonephropathy: a result of superimposed crescentic glomerulonephritis. J Am Soc Nephrol. 1995; 6:1541–1546.

3. Tse WY, Howie AJ, Adu D, Savage CO, Richards NT, Wheeler DC, et al. Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant. 1997; 12:1017–1027.

4. Kim TW, Park KK, Lee IH. A case of idiopathic membranous nephropathy with crescents formation associated with anti-gbm antibody. Korean J Nephrol. 2010; 29:628–633.
5. Lim SH, Kwon HH, Park KC, Ryu JI, You SS, Park MS, et al. A case of crescentric glomerulonephritis superimposed on preexisting membranous nephropathy. Korean J Nephrol. 2002; 21:820–825.
6. Haas M, Eustace JA. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int. 2004; 65:2145–2152.

7. Basford AW, Lewis J, Dwyer JP, Fogo AB. Membranous nephropathy with crescents. J Am Soc Nephrol. 2011; 22:1804–1808.

8. Cameron JS. Membranous nephropathy--still a treatment dilemma. N Engl J Med. 1992; 327:638–639.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download