Abstract
It is well known that patients with abdominal aortic aneurysm (AAA) often have concomitant coronary artery disease (CAD). In cases of AAA with severe CAD requiring coronary artery bypass grafting (CABG), two therapeutic strategies regarding the timing of CABG can be considered: staged or simultaneous operations. However, the ideal treatment of patients with large AAA and critical CAD remains controversial. We experienced a case of successful endovascular aneurysm repair after off-pump CABG in a 70-year-old patient who had a huge AAA and critical CAD.
Endovascular aneurysm repair (EVAR) is an important advance in the treatment of abdominal aortic aneurysm (AAA). Compared with open AAA repair, EVAR is associated with a significant reduction in perioperative mortality.1 Concurrent with the increased use of EVAR, a decrease in the incidence of ruptured AAA and associated morbidity and mortality has been reported, likely due to the ability to offer EVAR to patients who would not otherwise be candidates for open surgical repair.2 Some patients have features on the computed tomography (CT) scan that suggest "impending" rupture, and urgent or emergent AAA repair is generally indicated for these patients, provided the risk for repair is not prohibitive.3 EVAR is associated with lower perioperative morbidity and mortality compared with open surgical repair, but there is a risk that the endovascular repair may need to be converted to an open repair. Thus, patients should be evaluated and prepared as if undergoing an open surgical repair.4 It is well known that patients with AAA often have concomitant coronary artery disease (CAD),5 and cardiovascular events are a common cause of morbidity and mortality following AAA repair.6
Here we reported a case of successful EVAR after off-pump coronary artery bypass grafting (CABG) in a 70-year-old patient who had a large AAA and critical CAD.
A 70-year-old man presented with exertional dyspnea lasting 1 year. He had been on medication for heart failure through a local clinic, but his symptoms had become aggravated. On physical examination, holosystolic murmur was heard over the apex and a pulsatile mass was palpated in the lower abdomen. His electrocardiogram showed sinus rhythm with first-degree atrioventricular block, left atrial enlargement, and left bundle branch block (Fig. 1). The two-dimensional echocardiogram revealed a dilated left ventricular (LV) cavity with end-diastolic dimension of 67 mm, severe mitral regurgitation, and severe LV systolic dysfunction with ejection fraction of 25%. A 27-mm thrombus was noted in the LV apex (Fig. 2). Abdominal ultrasonography and CT angiography revealed a thrombosed aneurysmal dilatation of the infra-renal abdominal aorta extending to both the common and internal iliac arteries (maximum diameter up to 70 mm) (Fig. 3). Myocardial single-proton emission CT showed a fixed perfusion defect in the apex, anterior wall, and inferior wall (Fig. 4). The patient was put on intensive medical treatment for both ischemic cardiomyopathy with LV thrombus and AAA.
One week after anticoagulation with unfractionated heparin, a follow-up echocardiogram showed complete resolution of the apical thrombus. The patient underwent coronary angiography, which revealed near total occlusion of the middle left anterior descending artery, critical stenosis at the ostium of the left circumflex artery, and total occlusion of the distal right coronary artery (by nonselective ascending aortography due to an anomalous origin of the right coronary artery) (Fig. 5).
For AAA, EVAR was planned. Although EVAR is associated with lower perioperative morbidity and mortality compared with open surgical repair, CAD is known to be the leading cause of early and late mortality following AAA repair.5,6 Due to the patient's high-risk coronary anatomy, which was unsuitable for percutaneous coronary intervention, he underwent off-pump CABG. Simultaneously, he received mitral valve replacement surgery for severe ischemic mitral regurgitation. The two-dimensional echocardiogram after surgery showed improved LV systolic function and decreased LV chamber size (ejection fraction=37%, LV end-diastolic dimension=63 mm) with well-functioning tissue mitral valves.
Three weeks later, he underwent EVAR for AAA. After localization of both renal arteries, an abdominal aorta main body graft stent was deployed (25-16×145 mm Endurant stent; Medtronic, Inc., Minneapolis, MN, USA). Stenting for the right common iliac artery was performed by using a 16-24×120 mm Endurant stent. The final aortogram showed good expansion of the stents without leakage to the AAA (Fig. 6). The patient was discharged after 3 days of postprocedural care without any complications.
It is well known that patients with AAA often have concomitant CAD. The leading cause of perioperative mortality in AAA repair is cardiac related, and reduction of cardiac mortality has been considered to be important to improve outcomes.5,6 In cases of AAA with severe CAD requiring CABG, two therapeutic strategies regarding the timing of CABG can be considered, that is, staged or simultaneous operations.
In a staged operation, determining the priority order is a difficult decision. If the aneurysm repair is carried out initially, cross-clamping of the abdominal aorta increases systemic vascular resistance and left ventricular wall stress, leading to myocardial ischemia. Also, declamping induces a risk for myocardial ischemia following sudden hypotension. Thus, in a staged operation, open AAA repair after CABG is the gold standard.7 When CABG is carried out first, the inflammatory effects of cardiopulmonary bypass cause dilatation in the AAA, and the risk of AAA rupture during the perioperative period is elevated. To minimize this risk, CABG on the beating heart is preferred in both staged and simultaneous operations. But there are also potential risk factors in a staged operation in that patients undergo repeated anesthesia and two convalescence periods are needed.
To reduce the risks of repeat anesthesia and length of hospitalization, a one-stage operation consisting of both CABG and open repair of AAA should be preferred. However, a simultaneous operation also has potential dangers associated with elevated cardiac-related perioperative mortality following open AAA repair, especially in patients with diminished cardiac function.8,9 Also, the operating time of the combined operation is significantly longer than that of each single operation.
With recent advances in endovascular techniques, EVAR instead of open repair has rapidly expanded because it is relatively less invasive.8 In patients who have AAA with severe CAD, an operation carried out in a single session of EVAR and off-pump CABG been reported to be the optimal application. However, a specialized operating room and equipment are needed to perform this simultaneous procedure, and the fact remains that patients undergo EVAR with higher perioperative risk associated with severe CAD and diminished cardiac function.
In the present case, the patient had a huge AAA with impending rupture requiring repair. He was, however, at high risk of surgical complications owing to his multiple and severe CAD, which rendered him ineligible for percutaneous coronary intervention. To make matters worse, he also had severe systolic dysfunction with an enlarged chamber and severe valvular dysfunction.
First, we considered both a simultaneous operation, offpump CABG with open repair, and a staged operation, CABG after EVAR. However, because of the patient's diminished cardiac function and valvular dysfunction, we did not think that he would tolerate a prolonged operation time. Likewise, the latter was also dangerous owing to the high perioperative morbidity and mortality of AAA repair, especially in situations that convert to open repair. Finally, we planned EVAR after off-pump CABG with mitral valve replacement. Fortunately, the patient underwent successful off-pump CABG and mitral valve replacement, followed by timely EVAR with uneventful recovery. Also, the expected risk of EVAR is reduced through improved cardiac function. EVAR was performed under local anesthesia, so the patient underwent general anesthesia only once and had an additional convalescence period of just 3 days.
Although the ideal treatment of patients with large AAA and critical CAD remains controversial,10 the combination of off-pump CABG and EVAR may be the most promising treatment. However, optimal treatment must be individualized, and further studies are warranted to compare effectiveness between staged and simultaneous operations, in other words, EVAR after off-pump CABG versus EVAR with off-pump CABG simultaneously, especially in patients with severe CAD and diminished cardiac function. Furthermore, as for the staged operation, the optimal time interval between the two operations should be established, and the perioperative and long-term mortality of the combination of off-pump CABG and EVAR should be investigated.
Figures and Tables
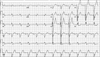 | FIG. 1Electrocardiography showed sinus rhythm with first-degree atrioventricular block, left atrial enlargement, and left bundle branch block. |
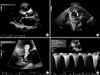 | FIG. 2Two-dimensional echocardiographic findings. Marked dilation of the LV chamber with severe LV systolic dysfunction (A) and a huge apical thrombus (B). Severe MR with PISA radius of 0.99 cm (C) and EROA of 0.5 cm2 (D). LV: left ventricle, MR: mitral regurgitation, PISA: proximal isovelocity surface area, EROA: effective regurgitant orifice area. |
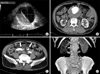 | FIG. 3Ultrasonographic and CT findings of AAA. A 76-mm abdominal aortic aneurysm with thrombosed false lumen shown by abdominal ultrasonography (A). Aneurysmal dilatation of the infra-renal abdominal aorta (B) and both thrombosed common iliac arteries (C) shown by CT angiography. Three-dimensional reconstructed image demonstrating aneurysmal dilatation infra-renal abdominal aorta extending to both the common and internal iliac arteries (D). AAA: abdominal aortic aneurysm, CT: computed tomography. |
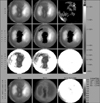 | FIG. 4Tc-99m MIBI myocardial SPECT showing fixed perfusion defect in the apex, anterior wall, and inferior wall. Tc-99m MIBI: technetium-99m methoxyisobutylisonitrile, SPECT: single-proton emission computed tomography. |
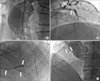 | FIG. 5Aortographic and coronary angiographic findings. Huge infra-renal abdominal aneurysm shown by aortogram (A). Near total occlusion in mLAD and critical stenosis in LCx-os (B) with collateral flow to RCA (C) and total occlusion in dRCA, visualized only by nonselective ascending aortogram due to its anomalous origin (D). mLAD: middle left anterior descending artery, LCx-os: ostium of the left circumflex artery, dRCA: distal right coronary artery. |
References
1. Jang MO, Kim JH, Oh SK, Lee MG, Park KH, Sim DS, et al. Endovascular stent in traumatic thoracic aortic dissection. Korean Circ J. 2012; 42:341–344.

2. Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009; 49:543–550.

3. Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009; 50:4 Suppl. S2–S49.

4. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011; 41:Suppl 1. S1–S58.

5. Hertzer NR, Beven EG, Young JR, O'Hara PJ, Ruschhaupt WF 3rd, Graor RA, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984; 199:223–233.
6. Cuypers PW, Gardien M, Buth J, Peels CH, Charbon JA, Hop WC. Randomized study comparing cardiac response in endovascular and open abdominal aortic aneurysm repair. Br J Surg. 2001; 88:1059–1065.

7. Paty PS, Darling RC 3rd, Chang BB, Lloyd WE, Kreienberg PB, Shah DM. Repair of large abdominal aortic aneurysm should be performed early after coronary artery bypass surgery. J Vasc Surg. 2000; 31:253–259.

8. de la Motte L, Jensen LP, Vogt K, Kehlet H, Schroeder TV, Lonn L. Outcomes after elective aortic aneurysm repair: a nationwide Danish cohort study 2007-2010. Eur J Vasc Endovasc Surg. 2013; 46:57–64.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download