Abstract
Generally, early exercise after coronary stenting is considered safe, but isolated cases of acute stent thrombosis have been associated with the performance of a treadmill exercise test after percutaneous coronary intervention (PCI). The treadmill exercise test is often used to noninvasively assess the functional result of PCI. In this report, we describe a case of terrible stent thrombosis related to an exercise test performed 3 days after stenting, and the patient died as the result of an intractable thrombus.
Generally, early exercise after coronary stenting is considered safe, as shown by some reports in the literature.1 However, several reports have described acute thrombotic occlusion associated with exercise testing shortly after successful percutaneous coronary intervention (PCI).2,3 Controversy exists regarding the appropriate timing of stress testing of patients who have undergone PCI. We report a case of subacute stent thrombosis (ST) associated with an exercise test conducted after PCI.
A 69-year-old man was referred to the cardiology department owing to chronic effort chest discomfort and abnormal findings of cardiac computed tomography angiography. He had lived in Argentina for 40 years and had a medical history of a previous percutaneous trans-femoral coronary angioplasty (PTCA) owing to stable angina in Argentina 16 years ago. However, he did not know which coronary artery was intervened in at that time. His chest pain had occurred 3 or 4 months ago, and he was in a typical pattern of stable angina that attacked him 2 to 3 times per day. He visited South Korea for a health examination and was first referred to the health screening center. His coronary risk factors were smoking and hypertension. The result of a computed tomography angiography showed chronic total occlusion of the right coronary artery (RCA), moderate stenosis of the left anterior descending artery (LAD), and high-grade stenosis of the left circumflex artery (LCX). The echocardiogram revealed a left ventricular ejection fraction of 62% by the modified Simpson's method and no regional wall motion abnormality.
The patient was admitted for coronary artery evaluation at our hospital and started to take aspirin and clopidogrel. His coronary angiography (CAG) showed total occlusion of the RCA with good collateral flow from the LAD (Fig. 1A) and significant stenosis of the LCX (Fig. 1B). Because he refused bypass surgery, coronary angioplasty was planned. PCI at the LCX lesion was done, and a 3.0×22 mm Endeavor Resolute stent (Medtronic, Minneapolis, MN, USA) was successfully deployed (Fig. 1C). After the PCI, the patient was prescribed aspirin 100 mg, clopidogrel 75 mg, nicorandil 10 mg, perindopril 4 mg, and rosuvastatin 10 mg. His compliance with the drugs was good. We performed a platelet function test using the verify-Now system and his value was normal at 486 ARU (reference range, <550) and 245 PRU (reference range, 1-386).
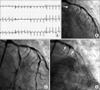
Because the patient was going to return to Argentina, we needed to establish treatment plans for the RCA chronic total occlusion, and we decided to perform a treadmill test by use of the modified Bruce protocol 3 days after the PCI. We wanted to determine if the patient's exercise capacity was tolerable without an additional procedure on the RCA lesion and whether any functional ischemia remained. During the treadmill test, the patient complained of chest pain in stage II and the electrocardiogram showed diffuse horizontal ST segment depression (Fig. 2A). Emergent CAG was performed and showed a thrombotic total occlusion of the proximal LCX stent that had been implanted 3 days before. After engaging the 6F EBU 3.5 (Medtronic) guiding catheter, we performed an urgent thrombectomy with an Export Thrombuster catheter (Medtronic). Subsequent CAG showed thrombus invasion up to the LAD ostium (Fig. 2B). Abruptly, the blood flow of the LAD slowed more and more and soon cardiogenic shock occurred. While performing cardiac pulmonary resuscitation, we injected abciximab through the intracoronary route and tried thrombus aspiration with the Export catheter several times. We could not achieve TIMI II or III flow until then. We performed PTCA at the LCX and LAD ostium several times. We eventually tried a crossover stent from the left main to the proximal LAD and finally succeeded in restoring complete LAD flow (Fig. 2C). Oddly, severe diffuse ST relapsed in the LAD, LCX, and left main after 5 minutes. We repeatedly tried ballooning, but in the end, intractable thrombi were found everywhere (Fig. 2D). Unfortunately, the patient died.
Generally, early exercise after PCI is considered safe.1 Roffi et al.1 performed a randomized trial including 1,000 patients to assess the safety of a symptom-limited exercise stress test the day after coronary stenting. In that study, exercise stress testing did not increase the risk of clinical ST. However, Roffi et al. excluded high-risk patients such as those with left main coronary artery disease or side vessel occlusion and reported one case of ST.
Several cases of acute thrombotic occlusion associated with exercise testing performed shortly after successful revascularization have been reported.2,3 Nevertheless, exercise testing is often tried to assess the functional result of PCI noninvasively. A number of reports have evaluated stress echocardiography after PCI for assessments of functional performance.4 In the meta-analysis cited above, the sensitivity and specificity of stress echocardiography for the detection of coronary stenosis after coronary revascularization were 82% and 86%, respectively.4
ST is a sudden thrombotic occlusion of a previously stented site. It has a dismal prognosis that results in sudden death or large myocardial infarction in most patients. Despite successful revascularization of ST, the 6-month mortality of patients with an ST complication is high. To date, the single most important cause of early and late ST is a patient's noncompliance with clopidogrel at the time of the event. Other important factors related to increased risk of both early and late ST include incomplete endothelialization of the vessel wall, incomplete stent expansion, residual plaque burden, small vessel caliber, left ventricular dysfunction, treatment of bifurcation lesions, and high platelet reactivity on treatment (oral antiplatelet therapy). The high platelet reactivity might be associated with polymorphisms in the genes that control the hepatic enzymes involved in clopidogrel metabolism.
It is important to note that the administration of dual anti-platelet therapy does not ensure that patients will not experience ST. Even though premature discontinuation of dual antiplatelet therapy is a risk factor for ST, many patients are on dual antiplatelet therapy at the time of the event. This was demonstrated in an observational study of over 10,000 patients undergoing sirolimus-eluting stent placement.5 Of those with early ST, 86% of patients were on dual anti-platelet therapy at that time. It is likely that multiple mechanisms explain the occurrence of ST, some of which cannot be prevented by dual anti-platelet therapy. In the present case, despite aspirin and clopidogrel administration, the patient developed early ST after the exercise test. The patient had risk factors for multi-vessel disease, but a direct cause of the ST in this case remains uncertain. His platelet function test value by use of the verify-Now system was normal. Generally, exercise training like cardiac rehabilitation is safe, and exercise training in ischemic cardiac disease has been shown to lower mortality.6 However, vigorous exercise might increase the risk of myocardial infarction.7 The pathophysiology of ST caused by excessive exercise is not completely understood. Coronary artery vasospasm, increased sympathetic activity, increased thrombin generation, increased vessel wall stress, and heightened platelet reactivity have been suggested to be involved.8 Also, there is evidence that moderate exercise and vigorous exercise have different effects on platelet function.9 Wang et al.9 showed that the influence of exercise training and deconditioning on platelet aggregation induced by alternating shear stress was probably through von Willebrand factor. In this case, our patient had not exercised for several years; therefore, abrupt exercise might have induced ST as a result of the mechanisms mentioned above. Even though he had RCA chronic total occlusion with good collateral flow from the LAD, the ST resulted in a tragic disaster.
The role of early exercise testing after PCI is challenged. Early exercise testing does not predict the occurrence of subacute ST or show any advantage in guiding future therapy. Also, AHA/ACC guidelines suggest a limited value of exercise testing in only high-risk patients and recommend exercise testing 3 to 6 months after PCI if the aim of exercise testing is to identify restenosis.10 Generally, 6 weeks is needed for stent endothelialization to occur after PCI. In another study evaluating the utility of exercise testing after PCI, the exercise test was safely performed at 6 weeks after PCI. Therefore, we think that exercise testing must be performed at least 6 weeks after coronary stenting and only when it is definitely necessary.
In conclusion, this was a serious case of ST intractable to current treatment modalities. What caused the ST in this case remains uncertain. It must be kept in mind that vigorous exercise tests can induce ST, and treadmill tests should therefore be done cautiously.
Figures and Tables
FIG. 1
Result of coronary angiography (CAG). (A) CAG showed total occlusion of the right coronary artery (RCA) with good collateral flow from the left anterior descending artery (LAD). (B) Significant stenosis of the left circumflex artery (LCX) was seen. (C) A 3.0×22 mm Endeavor Resolute stent was successfully placed in the LCX.

FIG. 2
(A) During the treadmill test, the patient complained of chest pain at stage II and the electrocardiogram accordingly showed diffuse horizontal ST segment depression. (B) Emergent coronary angiography (CAG) showed total occlusion of the left circumflex artery (LCX) stent by thrombosis and thrombus invasion up to the left anterior descending artery (LAD) ostium. (C) We tried a crossover stent from the left main to the proximal LAD and succeeded in completely restoring LAD flow. (D) Severe diffuse stent thrombosis relapsed in the LAD, LCX, and left main after 5 minutes. We repeatedly tried ballooning but intractable thrombi were found everywhere.
References
1. Roffi M, Wenaweser P, Windecker S, Mehta H, Eberli FR, Seiler C, et al. Early exercise after coronary stenting is safe. J Am Coll Cardiol. 2003; 42:1569–1573.

2. Samuels B, Schumann J, Kiat H, Friedman J, Berman DS. Acute stent thrombosis associated with exercise testing after successful percutaneous transluminal coronary angioplasty. Am Heart J. 1995; 130:1120–1122.

3. Nygaard TW, Beller GA, Mentzer RM, Gibson RS, Moeller CM, Burwell LR. Acute coronary occlusion with exercise testing after initially successful coronary angioplasty for acute myocardial infarction. Am J Cardiol. 1986; 57:687–688.

4. Dori G, Denekamp Y, Fishman S, Bitterman H. Exercise stress testing, myocardial perfusion imaging and stress echocardiography for detecting restenosis after successful percutaneous transluminal coronary angioplasty: a review of performance. J Intern Med. 2003; 253:253–262.

5. Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, Satler LF, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003; 108:43–47.

6. Newton M, Mutrie N, McArthur JD. The effects of exercise in a coronary rehabilitation programme. Scott Med J. 1991; 36:38–41.

7. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000; 343:1355–1361.

8. Hilberg T, Schmidt V, Gläser D, Schammne D, Lösche W, Gabriel HH. Platelet activity, sensitivity to agonist, and platelet--leukocyte conjugate formation after long-term exercise. Platelets. 2002; 13:273–277.

9. Wang JS, Li YS, Chen JC, Chen YW. Effects of exercise training and deconditioning on platelet aggregation induced by alternating shear stress in men. Arterioscler Thromb Vasc Biol. 2005; 25:454–460.

10. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002; 106:1883–1892.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download