Abstract
Percutaneous coronary intervention (PCI) of stumpless chronic total occlusion (CTO) lesions with a side branch stemming from the occlusion have a significantly lower treatment success rate because physicians cannot identify an accurate entry point with only conventional angiographic images. An intravascular ultrasonography (IVUS)-guided wiring technique might be useful for the penetration of stumpless CTO. We recently experienced thrombotic occlusion during an IVUS-guided stumpless CTO procedure. The cause of the thrombosis is not completely understood; the thrombosis may have been associated with the long use of the IVUS catheter. Special precautions should be taken to prevent thrombus in such cases.
Percutaneous coronary intervention (PCI) of chronic total occlusion (CTO) in coronary artery disease remains a major challenge for interventional cardiologists even in these times of technology advancement. Moreover, PCI of stumpless CTO lesions with a side branch stemming from the occlusion has a significantly lower success rate than does PCI of other hard lesions. This lower rate of success is because physicians cannot identify an accurate entry point with only conventional angiographic images and the guidewires tend to slip into a side branch. Several case reports have suggested that the intravascular ultrasound (IVUS)-guided wiring technique might be useful for penetration in cases of stumpless CTO. However, the safety of this method has not been sufficiently elucidated. We recently experienced diffuse thrombosis during an IVUS-guided stumpless CTO procedure. To our knowledge, diffuse thrombosis of CTO-PCI with IVUS has not been reported previously; thus, we present this case.
A 48-year-old man was admitted for the evaluation of coronary artery disease. A treadmill test had been performed 15 days previously to evaluate effort angina and exercise capacity and showed significant ST segment changes of a downward sloping 1-mm depression in II, III, aVF, and V4-V6. These findings developed at 2 minutes of stage 3 and persisted until the recovery stage.
Two years previously, the patient had undergone a coronary angiogram (CAG) owing to exertional chest pain. The CAG revealed that the right coronary artery (RCA) was in a chronic total occlusion state. No significant stenosis was shown in the left anterior descending artery (LAD) (Fig. 1A). PCI of the RCA was performed successfully with the result of TIMI flow 3. Since that time, the patient had been taking aspirin, clopidogrel, atorvastatin, beta-blocker, and angiotensin receptor blocker.
At admission, his blood pressure was 130/80 mmHg, his pulse rate was 78 per minute, and there were no specific laboratory findings. The PTT was 35.9 s and the PT INR was 1.0. He had neither protein C nor S deficiency. Electrocardiography showed Q wave at II, III, and V6, and echocardiography showed regional wall motion abnormality. A loading dose of 300 mg aspirin and 300 mg clopidogrel was administrated.
CAG findings revealed that the stent in the RCA was patent but the LAD showed stumpless CTO lesions with a side branch stemming from the occlusion from the LAD ostium; the collateral branch was supplied from the RCA (Fig. 1B). Because we had performed CAG before and knew the LAD pathway already, we did not perform coronary CT angiography. A guiding catheter (EBU 3.5, 7 Fr, Medtronic) was engaged for backup support by the femoral route. We first tried an antegrade approach without IVUS (I-Lab, Boston Scientific Corporation/SCIMed, Minneapolis, MN, USA). We routinely administer a bolus of 5000 units of heparin sulfate intravenously before both percutaneous transluminal coronary angioplasty and IVUS imaging.
After failure of wiring into the LAD several times, we decided to perform IVUS-guided wiring to find the optimal entry point and determine whether a guidewire properly penetrated the proximal cap. Run-through NS guidewires (Terumo, Tokyo, Japan) were advanced into the ramus intermedius to identify the LAD orifice through IVUS imaging while pulling back the catheter. The calcific LAD ostium and entry point were seen through IVUS. Consecutively, Filder XT (Asahi intecc, Osaka, Japan) and Miracle 3 and 6 g (Asahi intecc, Osaka, Japan) were used to penetrate the lesion under IVUS guiding. Attempts to bypass the lesion were made for 30 minutes with the aid of IVUS guidance. Abruptly, the patient's systolic blood pressure dropped below 70 mmHg, and diffuse thrombosis occurred at the ramus intermedius branch from the ostium to the distal segment. Ramus intermedius flow deteriorated to TIMI flow 1 (Fig. 1C). Immediately, while infusing intravenous dopamine and an intracoronary abciximab bolus injection, we removed the IVUS catheter and tried to remove the thrombus twice by use of an aspiration catheter (Export, Medtronics Inc, Minneapolis, MN, USA). After thrombo-suction, a large red thrombus was aspirated through the aspiration catheter (Fig. 2) and TIMI flow 3 returned (Fig. 1D). The next day, troponin-I was elevated from a baseline of 0.01 to 6.13 ng/ml and creatinine kinase-MB was elevated from 1.6 to 50.9 ng/ml. After a 2-day stay in the intensive care unit, the patient was discharged without any specific complications.
CTOs are encountered in approximately 20% of patients referred for coronary angiography.1 Several studies have documented that successful PCI of CTOs leads to an improvement in anginal symptoms, normalization of functional tests, improvement of LV function, and avoidance of coronary artery bypass graft surgery.2,3 Compared with other unfavorable lesions, stumpless CTO lesions with a side branch stemming from the occlusion have a significantly lower treatment success rate because physicians cannot identify an accurate entry point with only conventional angiographic images, and guidewires tend to slip into a side branch. The success rate of a CTO intervention relies on the morphologic characteristics of the lesion, but these morphologic characteristics are not well elucidated in vivo. IVUS remains the only routinely available intracoronary imaging modality; it provides tomographic images of the coronary artery.4
Park et al. reported in their prospective study including 31 patients with stumpless CTO lesions that 26 CTO lesions were successfully reopened (81%) with IVUS-guided wiring.5 In that report, procedure-related complications including coronary artery dissection and coronary artery perforation occurred in 8 lesions, but no events were serious. However, the safety of IVUS-guided wiring has still not been sufficiently elucidated.
The IVUS-guided wiring technique for a stumpless CTO lesion is known to be technically feasible and safe. But we must recognize that coronary artery perforation and dissection can occur as in other CTO procedures without IVUS guiding. Because CTO procedures are typically long and require the use of multiple intracoronary devices, prevention of intracoronary thrombosis and catheter thrombosis is important. Grayburn et al.6 reported a case that showed in vivo thrombus formation on a guidewire during IVUS imaging. We also experienced severe coronary artery thrombosis during an IVUS-guided stumpless CTO procedure. The cause of the thrombosis is not completely understood; the main cause might be the long presence of the IVUS catheter in the coronary artery despite the thrombogenicity of an intravascular catheter. The IVUS-guided wire was in place for a long time while we attempted to find the entry point and penetrate the CTO lesion. Also, thrombotic occlusion might have occurred as a result of insufficient control of the anticoagulants in this case. Unfortunately, we did not check the activated clotting time (ACT) right before and after the procedure or during the procedure. Therefore, we should have checked the ACT during PCI and administered an additional intravenous heparin injection. In addition, we used a 7-Fr guiding catheter. The 7-Fr guiding catheter might not have provided enough space to insert the IVUS catheter. We should have used an 8-Fr catheter and flushed the guiding catheter with saline more often because thrombus formation would have occurred in the guiding catheter. This case shows that we should pay attention to the occurrence of a thrombus if we leave the IVUS catheter in a coronary artery for a long time. Thrombus formation is one of the serious complications of PCI and should be prevented by sufficient anticoagulant control according to the patient's medical status and the appropriate selection of the guiding catheter.
Through this experience, we know that a long duration of use of an IVUS catheter for IVUS-guided wiring can cause diffuse thrombosis. Also, this case has meaning as the first case report of thrombosis caused by an IVUS-guided stumpless CTO procedure.
Figures and Tables
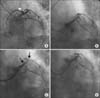
FIG. 1
Coronary angiographic findings during the PCI. (A) The intact left anterior descending artery (LAD) is shown 2 years previously by the white arrowhead in spider view. (B) At admission, the LAD is in a stumpless chronic total occlusion state with a side branch arising from the LAD ostium. (C) Diffuse thrombosis at the ramus intermedius 30 minutes after the IVUS-guided procedure. The thrombotic lesion is indicated by black arrows. (D) The final angiogram after thrombus aspiration and intracoronary abciximab bolus injection.
References
1. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012; 59:991–997.
2. Melchior JP, Doriot PA, Chatelain P, Meier B, Urban P, Finci L, et al. Improvement of left ventricular contraction and relaxation synchronism after recanalization of chronic total coronary occlusion by angioplasty. J Am Coll Cardiol. 1987; 9:763–768.

3. Warren RJ, Black AJ, Valentine PA, Manolas EG, Hunt D. Coronary angioplasty for chronic total occlusion reduces the need for subsequent coronary bypass surgery. Am Heart J. 1990; 120:270–274.

4. Fujii K, Ochiai M, Mintz GS, Kan Y, Awano K, Masutani M, et al. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006; 97:1455–1462.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download