Abstract
Stress cardiomyopathy (SCM) is usually precipitated by a physiologically or psychologically stressful event. Although it occurs only rarely, hypoxia- and hypercapnia-induced sympathetic activation may also cause SCM. We present the case of a 37-year-old woman affected with SCM after a routine colonoscopy. During the procedure, she aspirated residual polyethylene glycol from her stomach. Hypotension, resting dyspnea, and hemoptysis were subsequently observed. Laboratory findings revealed elevated cardiac enzymes, and a transthoracic echocardiogram revealed left ventricular (LV) global hypokinesia. She was ultimately diagnosed with diffuse alveolar hemorrhage-associated SCM. After successful treatment with a ventilator and corticosteroids, her LV systolic function and dimensions normalized and she was discharged without complications.
Stress cardiomyopathy (SCM) is an acute cardiac syndrome resembling ST-segment elevation myocardial infarction characterized by decreased wall motion in the apical and midportion of the left ventricle (LV). These abnormalities extend beyond a single coronary distribution without significant obstructive coronary artery disease.1 Case reports in the literature suggest that strong psychological or physical stress is associated with the occurrence of SCM.2 We believe that the present case is the first report of SCM following diffuse alveolar hemorrhage (DAH) owing to aspiration of polyethylene glycol (PEG).
A 37-year-old woman was transferred from a primary clinic to our emergency department because of chest pain and shortness of breath. She had undergone gastroscopy and colonoscopy in the left lateral decubitus position at the clinic that day to evaluate her complaint of constipation. During the examination, she aspirated some gastric contents with residual PEG. Although she received immediate first aid, her hypoxia did not resolve fully and her chest pain gradually worsened. At the time of her arrival at the emergency department, she complained of chest pain, shortness of breath, and blood-tinged sputum. She had no significant past medical history or medication history. Her initial vital signs were as follows: blood pressure, 80/50 mm Hg; heart rate, 114 beats/minute; respiration rate, 22 breaths/minute; body temperature, 37.2℃; and saturation, 95% in FiO2 0.32 by pulse oxymetry. Laboratory tests revealed a white blood cell count of 13,280/µl with 94.3% neutrophils and 0.3% eosinophils, hemoglobin of 13.0 g/dl, platelet count of 182,000/µl, activated partial thromboplastin time of 30.9 s, prothrombin time of 14.2 s, C-reactive protein of 2.10 mg/dl, significantly elevated troponin-I of 3.07 ng/ml, and elevated creatine kinase-MB of 15.7 ng/ml. The total creatine kinase level was not elevated at 194 U/L, but the creatine kinase-MB/creatine kinase ratio was markedly elevated at 8%. The electrocardiogram revealed sinus tachycardia without significant ST segment abnormality or T-wave inversion. A chest radiograph showed alveolar infiltration in the left upper lung field (Fig. 1A). Chest computed tomography showed ground-glass opacities and multiple low attenuation areas in the bilateral dependent portions, especially in the left upper lobe (Fig. 1C, D). The transthoracic echocardiogram revealed global hypokinesia, especially in the basal and midportions of the LV (Fig. 2). The LV ejection fraction was estimated to be approximately 30% by the A/L method, and there was no LV outflow tract obstruction, pulmonary hypertension, or mitral regurgitation. Initial pharmacologic management included aspirin, clopidogrel, and antibiotics based on the suspicion of acute coronary syndrome with aspiration pneumonia. The patient subsequently underwent cardiac catheterization. Coronary angiography revealed no significant stenosis of the major epicardial coronary arteries (Fig. 1E, F). Although left ventriculography and ergonovine provocation tests were not conducted, the typical echocardiography findings without significant coronary artery lesions were sufficient to confirm the diagnosis of SCM.
After the patient was transferred to the cardiac intensive care unit, her symptoms of hypoxia and dyspnea gradually worsened and fresh hemoptysis accompanied this worsening. While she was on 80% oxygen, her arterial blood gas measurements were as follows: pH, 7.306; PCO2, 26.7 mm Hg; PO2, 58.4 mm Hg; HCO3, 13.0 mmol/L; and SaO2, 88.2%. Ultimately, the patient was intubated, ventilator care was begun, and fiberoptic bronchoscopy was performed. No bronchial mucosal lesion was identified, but five sequential aliquots of bonchoalveolar lavage (BAL) fluid with a progressively bloody appearance were observed in the airway of the left upper area. Analysis of the BAL fluid revealed a red blood cell count of 28,000/mm3 and a white blood cell count of 100/mm3 with 40% neutrophils and 44% macrophages, consistent with alveolar hemorrhage. The patient received ventilator care for 3 days and was treated with methylprednisolone (starting dose of 1 mg/kg and tapering for 7 days to stop) and antibiotics. Seven days into her hospitalization, her symptoms of dyspnea, hemoptysis, and chest discomfort had fully resolved. Follow-up measurements of creatine kinase and creatine kinase-MB were normalized, and troponin-I was decreased to 0.13 ng/ml. The haziness on her chest radiograph had also disappeared (Fig. 1B). She was discharged without any further complications. Six months after her discharge from the hospital, a follow-up transthoracic echocardiogram revealed a 60% improvement in LV ejection fraction by the A/L method without regional wall motion abnormality (Fig. 3).
SCM is a reversible cardiomyopathy that is usually precipitated by a stressful condition. The pathophysiologic mechanism of SCM has not been clearly established, but a catecholamine-mediated mechanism has been recognized as the most reliable precipitating factor.3 DAH-induced hypoxia and hypercapnia have a multiplicative effect on the output of carotid chemoreceptors, and sympathetic activation is substantially increased.4 The exaggerated sympathetic responsiveness in DAH triggers myocardial stunning, multi-vessel epicardial spasm, microvascular spasm, and direct catecholamine-mediated myocyte injury, each of which has been reported to cause SCM.1 DAH-induced SCM could be explained in the same manner, but it has not previously been reported in the literature.
DAH refers to pulmonary hemorrhage originating from the alveolar capillaries, arterioles, and venules and is defined by the clinical symptoms of hemoptysis and anemia, diffuse radiographic pulmonary infiltration, and hypoxemic respiratory failure.5 DAH is characterized histologically by the presence of intraalveolar red blood cells, fibrin, and hemosiderin-laden macrophages, which may take up 48 to 72 hours to accumulate.5,6 Although a surgical biopsy specimen is considered the gold standard for diagnosis, it is often impractical. BAL is an accepted alternative for confirming the diagnosis when ≥20% hemosiderin-laden macrophages are present.7 According to one recent study, however, the percentage of hemosiderin-laden macrophages in BAL fluid can be increased in patients with DAH, but this may not be diagnostic.6 In our case, we performed BAL, but Prussian blue stained hemosiderin-laden macrophages were not present in the BAL fluid. Bronchoscopic examination was performed within 24 hours, which may have been too early in the course of the disease to detect the accumulation of hemosiderin-laden macrophages. The area of alveolar hemorrhage in this case was not extensive and the clinical course of our patient was relatively mild compared with other reported cases of DAH. This may be explained in part by the amount of hemosiderin-laden macrophages. Injury to the alveolar microcirculation is mainly associated with local lung injury or systemic disorders such as vasculitis or connective tissue disease.5 Our patient did not demonstrate any clinical evidence of a systemic disorder. Therefore, it seems more likely that local lung injury due to aspirated fluid followed by progression of acute respiratory distress syndrome was the cause of the DAH.
Although a wide range of DAH causes have been reported on a case-by-case basis, PEG exposure is a very rare etiology. PEG is a popular bowel cleansing agent because of the safety and efficacy of the solution; however, complications such as nausea, vomiting, and abdominal bloating are frequent.8 Some studies have reported esophageal rupture with vomiting or respiratory failure after PEG aspiration.9-11 The pathophysiological mechanism of PEG-induced lung injury remains unclear. According to some studies, the hyperosmotic properties of PEG solution can induce intra-luminary fluid shifting within the alveolar and interstitial spaces, resulting in pulmonary edema.12,13 In this case, massive lavage could retrieve a sufficient amount of aspirated fluid, especially PEG, preventing further deterioration of chemical pneumonitis and acute respiratory distress syndrome.
SCM and DAH have various underlying etiologies. However, PEG aspiration-induced DAH in young adults is very rare and only a few patients have recovered fully after retrieval of aspirated fluid with BAL. Furthermore, DAH-induced SCM has not previously been reported in the literature. The pathophysiological mechanism of PEG-induced lung injury is still not clear. Therefore, further studies examining the relationship between PEG and DAH are needed.
Figures and Tables
FIG. 1
Diagnostic images. (A) Chest radiograph showing multifocal alveolar infiltration; the left upper lung field is especially remarkable. (B) Chest radiograph after 7 days of treatment. (C, D) Chest computed tomography scans with ground-grass attenuation of both upper lobes and the left lower lobe. (E, F) Coronary angiograms indicating no significant stenosis of either coronary artery.
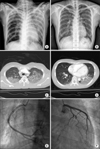
FIG. 2
Transthoracic echocardiogram at the time of hospitalization. Basal and midportions of the left ventricle are dilated and hypokinetic. (A, B) Parasternal long-axis view in the systolic and diastolic phase. (C, D) Parasternal short-axis view in the systolic and diastolic phases. (E) The M-mode recoding at the level of the papillary muscle showed a dilated and hypokinetic left ventricle.
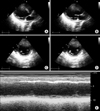
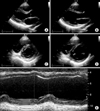
FIG. 3
Transthoracic echocardiogram 6 months after treatment. Left ventricular systolic function was fully recovered. (A, B) Parasternal long-axis view in the systolic and diastolic phases. (C, D) Parasternal short-axis view in the systolic and diastolic phases. (E) The M-mode recoding also revealed normal systolic function.
References
1. Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008; 124:283–292.

3. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012; 126:697–706.

4. Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995; 79:205–213.

6. Maldonado F, Parambil JG, Yi ES, Decker PA, Ryu JH. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur Respir J. 2009; 33:1361–1366.

7. Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002; 166:641–645.

8. Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum. 2006; 49:792–809.

9. Eisen GM, Jowell PS. Esophageal perforation after ingestion of colon lavage solution. Am J Gastroenterol. 1995; 90:2074.
10. Narsinghani U, Chadha M, Farrar HC, Anand KS. Life-threatening respiratory failure following accidental infusion of polyethylene glycol electrolyte solution into the lung. J Toxicol Clin Toxicol. 2001; 39:105–107.

11. de Graaf P, Slagt C, de Graaf JL, Loffeld RJ. Fatal aspiration of polyethylene glycol solution. Neth J Med. 2006; 64:196–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download