Abstract
The aim of this study was to compare the stent designed by Chonnam National University Hospital (designated as CNUH) with commercial cobalt-chromium coronary stent in a porcine coronary overstretch restenosis model. CNUH stent was subjected to mechanical performance tests. Pigs were randomized into two groups in which the coronary arteries (10 pigs, 10 coronaries in each group) had either CNUH stent or control commercial bare metal stent. Histopathologic analysis was assessed at 28 days after stenting. In mechanical performance tests, CNUH stent showed 2.65N, 35.1N, 0.52N, 1.94%, 4.29% in the flat plate radial compression, radial force, 3 point bending, Foreshortening and recoil test, respectively. There was no significant difference in the injury score, internal elastic lamina (IEL), lumen area, neointima area, percent area stenosis, inflammation score and fibrin score between the two groups (1.2±0.35, 4.1±0.41 mm2, 2.7±0.56 mm2, 1.6±0.47 mm2, 36.7±11.2%, 1.2±0.62, 0.2±0.34 in CNUH stent group vs. 1.2±0.38, 3.7±0.64 mm2, 2.5±0.49 mm2, 1.5±0.61 mm2, 36.3±12.17%, 1.1±0.12, 0.4±0.46 in commercial stent group, respectively). In the mechanical performance test, CNUH stent showed the moderated performance under the guideline of FDA. CNUH stent demonstrated similar histological reactions compared with commercial cobalt-chromium stent in a porcine coronary overstretch restenosis model.
Recently, Drug-eluting stents (DES) significantly reduce the rates of in-stent restenosis (ISR) and stent thrombosis by inhibiting neointimal tissue proliferation or thrombus formation compared with bare metal stents (BMS).1-3 Therefore, DES are used as suitable treatment for patients with myocardial infarction.4-6
However, DES may increase the risk of late in-stent thrombosis and delayed re-endothelialization.5,7-10 And recent developed BMS result in better outcome after coronary stent implantation.6,11-14 Newly designed bare metal coronary stent with excellent mechanical performance was designed by Chonnam National University Hospital (designated as CNUH), which is made of a cobalt-chromium based alloy that is stronger and more biocompatible than stainless steel.
Therefore we compared the novel CNUH stent with commercial cobalt-chromium coronary stent in a porcine coronary overstretch restenosis model.
To prepare CNUH stent, the Co-Cr tube (3.0 mm in a diameter) was cut and processed using laser cutting machine (StarCut Tube Femto, Hamburg, Germany). Thereafter, it was cleaned carefully using ultrasonic cleaning with acetone, ethanol and distilled water in sequence. To remove and crush of burr, it was applied in acidic atmosphere (50% H2SO4) for 1 hour. And then heat treatment and polishing process was performed to restore the mechanical properties and smoothen-surface of CNUH stent. The cleaned CNUH stents were kept under vacuum oven at 60℃ for 2 hours to evaporate the residual water (Fig. 1).
The successful deployment of stent is dependent on the clear understanding of its mechanical properties. CNUH stent was prepared and subjected to the radial compression test using both conventional flat plate and bearing and 3 point bending test, providing radial force and flexibility. Furthermore, it undertakes foreshortening and recoil test by expanding the stent.
The animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2012-34), and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Study animals were castrated male pigs weighing 20-25 kg. To prevent acute thrombosis after stenting, premedication with aspirin 100 mg and clopidogrel 75 mg per day was given for 5 days before the procedure. On the procedure day, pigs were anesthetized with zolazepam and tiletamine (2.5 mg/kg, Zoletil50®, Virvac, Caros, France), xylazine (3 mg/kg, Rompun®, Bayer AG, Leverkusen, Germany) and azaperone (6 mg/kg, Stresnil®, Janssen-Cilag, Neuss, Germany). They received supplemental oxygen continuously through oxygen mask. Subcutaneous 2% lidocaine at the cut-down site was administered, left carotid artery was surgically exposed, and a 7 French sheath was inserted.
Continuous hemodynamic and surface electrocardiographic monitoring were maintained throughout the procedure. Then 5,000 units of heparin was administered intravenously as a bolus prior to the procedure, the target coronary artery was engaged using standard 7 F guide catheters and control angiograms of both coronary arteries were performed using nonionic contrast agent in two orthogonal views.
The stent was deployed by inflating the balloon and the resulting stent-to-artery ratio was 1.3:1. Coronary angiograms were obtained immediately after stent implantation. Then, all equipment was removed and the carotid artery was ligated.
The 10 CNUH stents (3.0×18 mm) and 10 commercial stents (3.0×19 mm, Coroflex® Blue, B Brown, Mesungen, Germany) were implanted in the proximal left anterior descending artery and proximal left circumflex artery by randomized manner for 10 pigs. All received 100 mg of aspirin and 75 mg of clopidogrel daily until death.
Four weeks after stenting, the animals underwent follow-up angiography in the same orthogonal views before death with 20 ml of potassium chloride intracoronary injection.
The hearts were removed, and the coronary arteries were pressure-perfusion fixed at 110 mmHg in 10% neutral buffered formalin overnight. Arteries were step-sectioned, processed routinely for light microscopy, and stained for histological analysis.
Histopathologic evaluation of each artery was performed by an experienced cardiovascular pathologist. The specimens were embedded and sections of 50 to 100 µm thickness were obtained at about 1 mm apart and stained with Hematoxylin-Eosin for histological analysis (Fig. 2). Measurements of the histopathologic sections were performed using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech). Borders were manually traced for lumen area, area circumscribed by the internal elastic lamina, and the innermost border of the external elastic lamina (external elastic lamina area). Morphometric analysis of the neointimal area for a given vessel was calculated as the measured internal elastic lamina area minus lumen area. The measurements were made on five cross-sections from the proximal and distal ends and the three midpoints of each stented segment. Histopathologic stenosis was calculated as 100×[1-(lesion lumen area/lesion internal elastic lamina area)].15
Arterial injury at each strut site was determined by the anatomic structures penetrated by each strut. A numeric value was assigned, as previously described by Schwartz et al.15: 0=no injury; 1=break in the internal elastic membrane; 2=perforation of the media; 3=perforation of the external elastic membrane to the adventitia. The average injury score for each segment was calculated by dividing the sum of injury scores by the total number of struts at the examined section.
With regard to the inflammation score for each individual strut, the grading was as follows: 0=no inflammatory cells surrounding the strut; 1=light, non-circumferential lymphohistiocytic infiltrate surrounding strut; 2=localized, moderate to dense cellular aggregate surrounding the strut non-circumferentially; and 3=circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of struts at the examined section. Ordinal data for fibrin were collected on each stent section using a scale of 0 to 3 as previously reported (Fig. 3).16
Statistical analysis was performed with the aid of the commercially available software (SPSS Version 15, Chicago, IL, U.S.A.). The data were presented as mean value ± SD. Unpaired Student's t test was used for the comparison of the two stent groups. To examine the correlations between the measured histologic variables, regression analysis was applied for each set of measured variables. A value of p<0.05 was considered statistically significant.
Two stents were placed for two coronary arteries per swine. A total of twenty stents including ten CNUH stents and ten commercial stents, were placed in the proximal left anterior descending and proximal circumflex artery for ten swine. Mortality for this study was zero. There was no significant difference in stent-to-artery ratio between two stent groups.
In mechanical performance tests, CNUH stent showed 2.65N, 35.1N, 0.52N, 1.94%, 4.29% in the flat plate radial compression, radial force, 3 point bending, Foreshortening and recoil test, respectively. All experimental procedures followed the International Organization for Standardization (ISO) 25539-2 or a Standard Test Method (ASTM) F 2606-08.
In histopathological analysis, various amounts of inflammatory cells and fibrin infiltrate surrounding on each strut section. In neointima, most inflammatory cells were lymphohistiocytes in both groups. Carstair's fibrin stain for determining delayed arterial healing according to discriminate fibrin score that is not different between CNUH and commercial stent group.
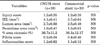
There was no significant difference in the injury score between the two groups (1.2±0.35 in CNUH stent group vs. 1.2±0.38 in commercial stent group, p=0.82). There was no significant difference in the internal elastic lamina (IEL) between the two groups (4.1±0.41 mm2 in CNUH stent group vs. 3.7±0.64 mm2 in commercial stent group, p=0.06). There was no significant difference in the lumen area between the two groups (2.7±0.56 mm2 in CNUH stent group vs. 2.5±0.49 mm2 in commercial stent group, p=0.19). There was no significant difference in the neointima area between the two groups (1.6±0.47 mm2 in CNUH stent group vs. 1.5±0.61 mm2 in commercial stent group, p=0.55). There was no significant difference in the percent area stenosis between the two groups (36.7±11.2% in CNUH stent group vs. 36.3±12.17% in commercial stent group, p=0.88). There was no significant difference in the inflammation score between the two groups (1.2±0.62 in CNUH stent group vs. 1.1±0.12 in commercial stent group, p=0.22). There was no significant difference in the fibrin score between the two groups (0.2±0.34 in CNUH stent group vs. 0.4±0.46 in commercial stent group, p=0.06) (Table 1, Fig. 4).
A bare metal coronary stent with novel design was developed at Chonnam National University Hospital (CNUH). In the mechanical performance test, CNUH stent showed the moderated performance under the guideline of FDA. CNUH stent demonstrated similar histological reactions compared with commercial cobalt-chromium stent in a porcine coronary overstretch restenosis model.
Although bare metal stent have higher rate of restenosis than drug eluting stent, bare metal stent is better in patients with bleeding disorder. CNUH stent, showed the sufficient performance under the guideline of FDA and will be used in patients with bleeding risk and platform for development of newly concept coronary stents such as drug elution, gene delivery and nanoparticle transport.
Commercial stent, was used in this experiment, the Coroflex® Blue is a new low-profile, non-ferromagnetic stent with a thin strut thickness of 65 µm. The stent is made of a cobalt-chromium based alloy which is more solid and harder than 316 L stainless steel, allowing for better deliverability, increased flexibility, and thinner struts without compromising radio-opacity or radial strength. Coroflex® Blue registry included 2,315 patients (mean age 64.3±11.1 years, 19.8% diabetes, 37.3% acute myocardial infarction) demonstrated the efficacy and safety of the cobalt-chromium stent in real world practice.17
Cobalt-chromium stents have shown lower target lesion revascularization (TLR) -rates than stainless steel stents in a limited number of patients with selected indication for percutaneous coronary intervention (PCI). In registry studies, the TLR-rate for the cobalt-chromium stent in 298 patients was 8.8% after 9 months.18
The implantation of coronary stent with the same material but with varying design is associated with a significantly different result in patients. Although the 30 day outcome was not significantly different, both 6 month angiographic and 1 year clinical results were significantly different.11
Coronary stent with thin strut thickness may reduce the restenosis rate in coronary artery with a reference diameter <3.0 mm.13 Commercial stent strut thickness (65 µm) is thinner than CNUH stent (80 µm). We will produce a thinner thickness of cobalt-chromium coronary stent.
In our study, CNUH stent showed the moderated performance under the guideline of FDA in the mechanical performance test. Moreover CNUH stent demonstrates satisfactory hitopathological reaction compared with commercial stent.
Signs of delayed arterial healing were significantly greater with increased fibrin score.19 In the fibrin score, CNUH stents tended to be lower compared to commercial stents without statistically significant (p=0.06). However, both stent showed very low fibrin score.
Our previous studies which were coated by various drugs (heparin, carvedilol, probucol, alpha lipoic acid, ramiprilat, abciximab) on bare metal stents demonstrated inhibition of neointimal hyperplasia, restenosis and acute thrombosis in porcine coronary restenosis model.20-24 Moreover, we showed that abciximab-coated stents was safe and effective in ninety-six patient with acute myocardial infarction.25
In order to overcome the limitations of drug coating stent using polymer, we apply various methods. The previous study used dopamine to immobilized heparin on a stent surface showed thromboresistant and endothelialization effects in rabbit iliac artery restenosis model.26 And a TiO2 and nitrogen doped-TiO2 thin film was deposited by plasma enhanced chemical vapor deposition (PECVD) process and its applicability as a drug coating matrix was demonstrated in promising alternatives to polymer for the preparation of drug-eluting stents.27,28 In addition, to accelerate re-endothelialization response of the bare metal stent, the aptamer stent was specifically coated by oligonucleotides functioning as endothelial progenitor cell (EPC)-attracting messenger.29
Recently, we showed that Akt1 siRNA/ssPEI nanoparticles released from the hyaluronic acid coated stent suppressed the neointima hyperplasia, resulting in the prevention of restenosis in rabbit model.30
Although our developed stent is not significant superior compared with commercial stent, the significance of CNUH stent is that we have core technology on our own bare metal stents with originality.
We had used commercial bare metal stents in previous studies. Because our CNUH stent shows sufficient effects, however, we will use our bare metal stent in novel coronary stent experiments.
In the mechanical performance test, CNUH stent showed the moderated performance under the guideline of FDA. CNUH stent demonstrated similar histological reactions compared with commercial cobalt-chromium stent in a porcine coronary overstretch restenosis model. This newly designed CNUH stent will be used for stent platform for new drug-eluting and gene-drug delivery stents in the future.
Figures and Tables
FIG. 1
Design plan of CNUH stent (A). Scanning electron micrographs of CNUH stent surface (B, ×20; C, ×1,000; D, ×2,000).

FIG. 2
Representive images of H&E staining after 4 weeks of stenting. Specimen CNUH stent implanted (A, ×20) and commercial stent implanted (B, ×20).

FIG. 3
The Carstair fibrin stain of the low-power fields (magnitude, ×20) of fibrin infiltration in CNUH stent (A) and commercial stent (B).

ACKNOWLEDGEMENTS
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2012-0001047).
References
1. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.

2. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004. 350:221–231.

3. Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention. 2005. 1:58–65.
4. Gupta M, Budoff MJ. Drug-eluting stent versus bare-metal stent in acute ST-segment elevation myocardial infarction: a word of caution. J Invasive Cardiol. 2010. 22:159–160.
5. Kaltoft A, Kelbaek H, Thuesen L, Lassen JF, Clemmensen P, Kløvgaard L, et al. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2010. 56:641–645.
6. Di Lorenzo E, Sauro R, Varricchio A, Capasso M, Lanzillo T, Manganelli F, et al. Benefits of drug-eluting stents as compared to bare metal stent in ST-segment elevation myocardial infarction: four year results of the PaclitAxel or Sirolimus-Eluting stent vs bare metal stent in primary angiOplasty (PASEO) randomized trial. Am Heart J. 2009. 158:e43–e50.

7. Vorpahl M, Yazdani SK, Nakano M, Ladich E, Kolodgie FD, Finn AV, et al. Pathobiology of stent thrombosis after drug-eluting stent implantation. Curr Pharm Des. 2010. 16:4064–4071.

8. Kim W, Jeong MH, Hwang SH, Kim KH, Hong YJ, Ahn YK, et al. Comparison of abciximab combined with dalteparin or unfractionated heparin in high-risk percutaneous coronary intervention in acute myocardial infarction patients. Int Heart J. 2006. 47:821–831.

9. Ma GT, Li L, Wu XH. ST-elevation myocardial infarction caused by very late stent thrombosis due to drug-eluting stent fracture. Chin Med J (Engl). 2012. 125:2794–2796.
10. de la Torre Hernández JM, Windecker S. Very late stent thrombosis with newer drug-eluting stents: no longer an issue? Rev Esp Cardiol (Engl Ed). 2012. 65:595–598.

11. Kastrati A, Dirschinger J, Boekstegers P, Elezi S, Schühlen H, Pache J, et al. Influence of stent design on 1-year outcome after coronary stent placement: a randomized comparison of five stent types in 1,147 unselected patients. Catheter Cardiovasc Interv. 2000. 50:290–297.

12. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, et al. Intracoronary stenting and angiographic results strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Vestn Rentgenol Radiol. 2012. (2):52–60.
13. Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, et al. In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002. 40:403–409.
14. Douglas G, Van Kampen E, Hale AB, McNeill E, Patel J, Crabtree MJ, et al. Endothelial cell repopulation after stenting determines in-stent neointima formation: effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J. 2012. [Epub ahead of print].

15. Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.

16. Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008. 1:143–153.
17. Bocksch W, Pomar F, Dziarmaga M, Tresukosol D, Ismail O, Janek B, et al. Coroflex Blue Registry Investigators. Clinical safety and efficacy of a novel thin-strut cobalt-chromium coronary stent system: results of the real world Coroflex Blue Registry. Catheter Cardiovasc Interv. 2010. 75:78–85.

18. Sketch MH Jr, Ball M, Rutherford B, Popma JJ, Russell C, Kereiakes DJ. Driver Investigators. Evaluation of the Medtronic (Driver) cobalt-chromium alloy coronary stent system. Am J Cardiol. 2005. 95:8–12.

19. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006. 48:193–202.
20. Lim KS, Hong YJ, Hachinohe D, Ahmed K, Jeong MH, Kim JH, et al. Effect of a dual drug-coated stent with abciximab and alpha-lipoic Acid in a porcine coronary restenosis model. Korean Circ J. 2011. 41:241–247.

21. Hong YJ, Jeong MH, Song SJ, Sim DS, Kim JH, Lim KS, et al. Effects of ramiprilat-coated stents on neointimal hyperplasia, inflammation, and arterial healing in a porcine coronary restenosis model. Korean Circ J. 2011. 41:535–541.

22. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Cho JG, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.

23. Lim SY, Bae EH, Jeong MH, Kim JH, Hong YJ, Sim DS, et al. Effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009. 54:375–385.
24. Kim W, Jeong MH, Cha KS, Hyun DW, Hur SH, Kim KB, et al. Effect of anti-oxidant (carvedilol and probucol) loaded stents in a porcine coronary restenosis model. Circ J. 2005. 69:101–106.

25. Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: ReoPro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006. 47:933–938.

26. Bae IH, Park IK, Park DS, Lee H, Jeong MH. Thromboresistant and endothelialization effects of dopamine-mediated heparin coating on a stent material surface. J Mater Sci Mater Med. 2012. 23:1259–1269.

27. Song SJ, Jung KW, Park YJ, Park J, Cho MD, Jeong MH, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparationof drug-eluting stents. J Mater Chem. 2011. 21:8169–8177.

28. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2009. 20:4792–4801.

29. Sim DS, Kwon JS, Kim YS, Chung HC, Hong YJ, Park HW, et al. Experience with endothelial progenitor cell capturing aptamers for coating of intracoronary stents in a porcine model. Tissue Eng Regen Med. 2009. 6:555–561.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download