Abstract
In contrast to widely recognized venous thrombotic complications, peripheral arterial thrombosis as a complication of nephrotic syndrome, especially without preceding iatrogenic venous puncture, corticosteroid treatment, or coagulation factor abnormalities, has rarely been reported in adult female patients. We report the case of a 39-year-old woman who presented with pain in the right lower leg accompanied by minimal change nephrotic syndrome. Lower-extremity angiography showed total occlusion of the right superficial femoral artery. Thrombectomy was performed with a balloon catheter, and the thrombi were successfully aspirated. Our experience indicates that even if few traditional risk factors for atherosclerosis are identified, a high index of suspicion and aggressive treatment of arterial thrombosis in adult nephrotic syndrome are crucial to minimize serious ischemic injuries.
Hypercoagulability-induced thromboembolism is a serious complication of nephrotic syndrome and often leads to grave consequences. Although venous thromboembolic complications including deep vein thrombosis, pulmonary embolism, and, in particular, renal vein thrombosis are relatively common and well recognized,1 peripheral arterial thrombosis has rarely been reported in the literature. Furthermore, reports of femoral arterial thrombosis are even more uncommon and are almost exclusively described in nephrotic children with iatrogenic vascular injury or corticosteroid treatment.2 Additionally, once developed, nephrotic syndrome-associated arterial thrombosis has been reported to cause high rates of limb loss, neurologic sequelae, and even patient death.2,3 Thus, we present here a case of femoral artery thrombosis associated with minimal change nephrotic syndrome in an adult female patient that was not preceded by iatrogenic venipuncture or the use of corticosteroids. The case was successfully treated with thrombectomy and oral anticoagulation without vascular sequelae.
A previously healthy, 39-year-old woman was admitted to our hospital with generalized edema lasting 1 month and with sudden-onset pain in her right lower leg, particularly when walking. On admission, her general appearance was acutely ill and generally edematous. A physical examination revealed that her blood pressure was 120/80 mmHg, her heart rate was 90/min, her respiratory rate was 22/min, and her body temperature was 36.5℃. Her breathing sounds were clear, and her heartbeat was regular without murmur. A subsequent examination of her lower limbs showed asymmetric bilateral pretibial pitting edema predominantly in the left lower limb. In contrast, the right lower limb was less edematous and had very weak popliteal and pedal pulses. Delayed capillary refilling was also found in the right toes. The Homan's sign was negative.
Serum laboratory findings at admission were as follows: white blood cell count of 5,300/mm3, hematocrit of 39.5% with hemoglobin of 13.1 g/dl, and platelet count of 286,000/mm3. The serum sodium concentration was 141 mEq/L, the potassium concentration was 4.1 mEq/L, the chloride concentration was 104 mEq/L, and total CO2 was 28 mEq/L. The blood urea nitrogen concentration was 12.1 mg/dl, the creatinine concentration was 0.86 mg/dl, the serum total protein concentration was 4.3 g/dl, the albumin concentration was 2.2 g/dl, the total cholesterol concentration was 349.4 mg/dl, the low-density lipoprotein cholesterol concentration was 214.4 mg/dl, the high-density lipoprotein cholesterol concentration was 111 mg/dl, and the triglyceride concentration was 120.1 mg/dl. The results of liver function tests were normal. The coagulation profile showed an activated partial thromboplastin time of 32.0 s, prothrombin time of 9.7 s, anti-thrombin III value of 82% (70-120%), protein C activity of 143% (70-130%), and protein S activity of 65% (58.7-119.2%). The anti-nuclear antibody titer was less than 1:40, the anti-double-stranded DNA titer was 30.9 IU/ml, the C3 level was 158 mg/dl, and the C4 level was 19 mg/dl. Anti-cardiolipin immunoglobulin G, anti-cardiolipin immunoglobulin M, and lupus anticoagulant were all negative. Urinalysis revealed 3+ proteinuria (spot urine protein-to-creatinine ratio was 10.9 g/g creatinine; spot urine albumin-to-creatinine ratio was 7,406.2 mg/g creatinine) and trace hematuria (1-3 red blood cells/high-power field). To investigate the etiology of nephrotic syndrome, an urgent renal biopsy was performed.
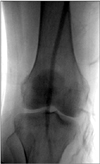
Thereafter, although she had no risk factors for thrombosis such as immobilization, heart failure, atrial fibrillation, morbid obesity, or a recent orthopedic or gynecologic surgery, to rule out arterial thrombosis of the right lower extremity, lower extremity computed tomography (CT) angiography was performed. The CT imaging revealed that the right superficial femoral artery was occluded and there was no identifiable proximal source of thromboembolism in other large arteries (Fig. 1). On the basis of these CT angiographic findings, right lower extremity angiography was performed, which revealed a total occlusion of the right superficial femoral artery (Fig. 2). For the treatment of this condition, balloon dilatation was initially tried at the distal superficial femoral artery but failed. Hence, thrombectomy was performed with the Fogarty balloon catheter and the thrombi were successfully aspirated. After the interventional treatment, a good distal arterial flow was reestablished (Fig. 3). Thereafter, warfarin (5 mg/day) as an anticoagulant and corticosteroid treatments were initiated.
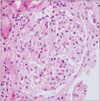
Subsequent transthoracic and transesophageal echocardiographic studies showed no evidence of intracardiac thrombus, vegetation, or other structural abnormalities including patent foramen ovale. Quantitation of proteinuria was confirmed by using a 24-hour urine collection that showed 10.2 g/day of protein excretion and 7.8 g/day of albumin excretion, respectively. Afterwards, with administration of warfarin and corticosteroid, the patient had nearly completely recovered from the edema and claudication and was discharged. The renal pathologic results were consistent with minimal change disease (Fig. 4 and Fig. 5). Two months after discharge, her urine protein-to-creatinine ratio decreased to less than 0.1 g/g creatinine, her serum albumin level was maintained at 4.1 g/dl, and she had no further symptoms or signs of arterial thrombosis.
Arterial thrombotic events involving the lower extremities in adults are much rarer than venous thrombotic events, and only a few cases have been reported in the English literature. According to Nishimura et al.'s review, only seven male cases had been reported up through 2000.4
Compared with the cases described in this review,4 several points can be noted in the present case. First, to the best of our knowledge, ours is the only case in the English literature of nephrotic syndrome-associated arterial thrombosis in the lower limb in an adult female patient, especially a patient without preceding iatrogenic venipuncture or use of corticosteroids. What explains this huge between-gender difference in the occurrence of arterial thrombosis in nephrotic syndrome? Although the mechanism is unclear, a few observations support the phenomenon in both nonnephrotic and nephrotic patients. Arterial thrombi are primarily composed of platelets with small amounts of fibrin, which contrasts sharply with venous thrombi, which are composed largely of fibrin and trapped erythrocytes. In this context, the role of altered platelet activity in nonnephrotic patients with arterial thrombosis was tested, and it was found that dysfunctional platelet retention was significantly more prominent in males than in females.5 Furthermore, a significantly preferential occurrence of arterial thrombotic events defined by broader criteria including myocardial infarction and ischemic stroke was also reported in male nephrotic patients.6 Second, the arterial thrombotic event in this case was preceded neither by corticosteroid therapy nor by iatrogenic vessel trauma, which was commonly observed in previous case reports.2,7 Third, in contrast with a previous report,4 the levels of anticoagulation factors were within the normal range. Thus, anticoagulation factor deficiency is less likely to have mediated the event in this case. Finally, it was noted that most of the patients in the reported cases of lower limb arterial thrombosis had seriously poor outcomes. According to the summary of seven cases by Nishimura et al.,4 one patient died, three patients underwent an amputation of the affected limb, and two of these three also sustained recurrent thrombotic events. In contrast, in the present case, early detection and urgent interventional management of the arterial thromboembolic lesion and subsequent immediate anticoagulation might enable the limb to be salvaged and protected against further ischemic injury.
The factors predisposing to arterial thrombosis in patients with nephrotic syndrome are poorly defined. It is postulated that the degree of hypoalbuminemia (particularly, <2.0 g/dl) correlates with lower levels of endogenous anticoagulant antithrombin, and this might predispose to an increased incidence of thromboembolism.8,9 Furthermore, the optimal indications for prophylactic antiplatelet or anticoagulation therapy against arterial thrombosis are also unclear to date, as are the indications for prophylactic therapy against thromboembolic complications of venous origin.10
In conclusion, arterial thrombosis is a potentially grave problem in nephrotic patients. This report highlights that clinicians should maintain a high index of suspicion if nephrotic patients present symptoms suggesting acute peripheral arterial occlusion, even if the patients have few traditional risk factors for atherosclerosis. Furthermore, urgent, aggressive management of arterial thrombosis is mandated to minimize serious morbidities associated with progression of ischemic injury.
Figures and Tables
FIG. 1
Lower-extremity CT angiography showed occlusion of the right popliteal artery and the ostial portions of the anterior tibial, posterior tibial, and peroneal arteries.
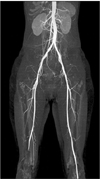
FIG. 2
The right lower-extremity arteriogram showed a complete occlusion with a filling defect of the superficial femoral artery.
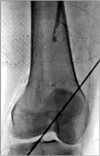
FIG. 3
The second arteriogram of the right lower extremity after thrombectomy. Revascularized distal superficial femoral artery and a good distal arterial flow were observed.
References
1. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006. 118:397–407.

2. Tarry WC, Moser AJ, Makhoul RG. Peripheral arterial thrombosis in the nephrotic syndrome. Surgery. 1993. 114:618–623.
3. Khatri VP, Fisher JB, Granson MA. Spontaneous arterial thrombosis associated with nephrotic syndrome: case report and review of the literature. Nephron. 1995. 71:95–97.

4. Nishimura M, Shimada J, Ito K, Kawachi H, Nishiyama K. Acute arterial thrombosis with antithrombin III deficiency in nephrotic syndrome: report of a case. Surg Today. 2000. 30:663–666.

5. Lilienberg G, Venge P. Platelet adhesion in patients prone to arterial and venous thrombosis: the impact of gender, smoking and heredity. Scand J Clin Lab Invest. 1998. 58:279–286.

6. Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008. 117:224–230.

7. Sullivan MJ 3rd, Hough DR, Agodoa LC. Peripheral arterial thrombosis due to the nephrotic syndrome: the clinical spectrum. South Med J. 1983. 76:1011–1016.
8. Tarry WC, Moser AJ, Makhoul RG. Peripheral arterial thrombosis in the nephrotic syndrome. Surgery. 1993. 114:618–623.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download