Abstract
The effects of statins on insulin resistance and new-onset diabetes are unclear. The purpose of this study was to evaluate the effects of rosuvastatin on insulin resistance and adiponectin in patients with mild to moderate hypertension. In a randomized, prospective, single-blind study, 53 hypertensive patients were randomly assigned to the control group (n=26) or the rosuvastatin (20 mg once daily) group (n=27) during an 8-week treatment period. Both groups showed significant improvements in systolic blood pressure and flow-mediated dilation (FMD) after 8 weeks of treatment. Rosuvastatin treatment improved total cholesterol, low-density lipoprotein (LDL)-cholesterol, and triglyceride levels. The control and rosuvastatin treatment groups did not differ significantly in the change in HbA1c (3.0±10.1% vs. -1.3±12.7%; p=0.33), fasting glucose (-1.3±18.0% vs. 2.5±24.1%; p=0.69), or fasting insulin levels (5.2±70.5% vs. 22.6±133.2%; p=0.27) from baseline. Furthermore, the control and rosuvastatin treatment groups did not differ significantly in the change in the QUICKI insulin sensitivity index (mean change, 2.2±11.6% vs. 3.6±11.9%; p=0.64) or the HOMA index (11.6±94.9% vs. 32.4±176.7%; p=0.44). The plasma adiponectin level increased significantly in the rosuvastatin treatment group (p=0.046), but did not differ significantly from that in the control group (mean change, 23.2±28.4% vs. 23.1±27.6%; p=0.36). Eight weeks of rosuvastatin (20 mg) therapy resulted in no significant improvement or deterioration in fasting glucose levels, insulin resistance, or adiponectin levels in patients with mild to moderate hypertension.
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are prescribed worldwide in patients with or at risk for cardiovascular disease (CVD). Reduction of low-density lipoprotein (LDL) cholesterol is one of the primary mechanisms of CVD prevention. Beyond the lipid-lowering effect of statins alone, there is abundant evidence showing that statins provide immediate benefits, the so-called pleiotropic effects of statins. These pleiotropic effects are thought to include improved endothelial function, enhanced stabilization of atheromatous plaque, decreased oxidative stress, decreased vascular inflammation, and a decrease in the probability of developing atherosclerotic events in metabolic syndrome, type 2 diabetes, and hypertension.1-6 These effects of statins may consequently prevent plaque rupture and subsequent myocardial infarction in the proinflammatory and prothrombotic environment.7,8 Recently, randomized controlled clinical trials have raised the concern that lipophilic statins might have unfavorable metabolic effects, such as reducing insulin secretion and exacerbating insulin resistance and the development of new-onset diabetes.3,9,10 Another study also showed that atorvastatin treatment resulted in significant increases in fasting insulin and glycated hemoglobin (HbA1C) levels consistent with insulin resistance in hypercholesterolemic patients.11 These concerns are very important because insulin resistance increases the risk of CVD. Although some studies have been published on the adverse effects of statins, their effects on insulin resistance and new-onset diabetes are not obvious.3,6,11,12
The purpose of this study was to evaluate the effects of rosuvastatin on insulin resistance and adiponectin in patients with newly diagnosed mild to moderate hypertension.
This study was a randomized, prospective, single-blind study in patients with mild to moderate hypertension [systolic blood pressure (BP)<170 mmHg or diastolic BP<105 mmHg] from September 2009 to April 2010. The study was carried out in Gwangju Veterans Hospital and was approved by the institutional review board of the hospital. Every patient was given full information about the study objectives and methods and signed a written informed consent form. No patient had taken any lipid-lowering agent, hormone therapy, or vitamin supplements during the 8 weeks before randomization. Also, during the pre-randomization period (8 weeks) and the study period, to make the comparison of insulin sensitivity fair in the two groups, all patients took an angiotensin type II receptor blocker (ARB), telmisartan 80 mg, followed by a calcium channel blocker for the treatment of hypertension. Patients with newly diagnosed mild to moderate hypertension were included. We excluded patients with renal disease, hepatic disease, any thyroid disease, uncontrolled diabetes (HbA1C>8%), uncontrolled severe hypertension, stroke, acute coronary syndrome, and unstable angina.
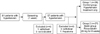
After a 1-week screening period, 57 patients were randomly assigned to either placebo (Group I: mean, 61.5±6.9 years, n=26) or rosuvastatin 20 mg (Group II: mean, 60.4±7.2 years, n=27) once daily during a 2-month treatment period. The allocation was performed by using envelopes. At screening, 57 patients were enrolled in the study. One patient was diagnosed with hepatocellular carcinoma. Three patients withdrew their informed consent. Thus, the final analysis was performed on 53 patients (Fig. 1).
The patients were examined at baseline and at 8-week follow-up visits to assess changes in fasting glucose, insulin, HbA1C levels, QUICKI (quantitative insulin-sensitivity check index), HOMA (homeostasis model assessment), adiponectin, and flow-mediated vasodilation (FMD).
For BP measurement, stabilization was attempted for more than 10 minutes. BP was measured on the right upper arm with the patient in a sitting position. The measurement was performed at least 2 times at a minimum interval of 10 minutes and the measurements were averaged. Systolic BP of more than 140 mmHg or diastolic pressure of more than 90 mmHg was defined as hypertension.
The evaluation of vascular endothelial function was performed by FMD, a noninvasive method. To ensure that the ultrasonographic findings of the brachial artery were detected, the most accessible area, which was 2 to 5 cm inferior to the antecubital fossa, was targeted by use of a high-resolution ultrasonography unit (Sequoia 512; Acuson, Mountain View, CA, USA) to which a 10 MHz linear array transducer was implanted. Ultrasonography was performed according to methods reported previously.13,14
Blood sampling was done in the morning before treatment and after 8 weeks of drug administration and more than 8 hours of fasting. Plasma insulin was measured with a radioimmunoassay (Biosource Inc., Nivelles, Belgium), as was adiponectin (LINCO Research Inc., St. Louis, MO, USA). Indices for insulin sensitivity (QUICKI and HOMA) were calculated on the basis of the following formulas: QUICKI=1/{log (insulin)+log (glucose)} and HOMA=fasting insulin × fasting glucose/22.5. The units of measurement of insulin and glucose were µU/ml and mg/dl, respectively.
All data are expressed as the mean±SD. We used Student's paired t test or Wilcoxon signed rank test to compare values between baseline and treatment at 2 months. A comparison of the measurements between the two groups was made by using repeated-measures ANOVA. The mean delta change (%) was calculated as a mean of delta change=(baseline value - follow-up value)/baseline value × 100 (%). All statistical procedures were performed with the Statistical Package for the Social Sciences (SPSS), version 13.0 (SPSS Inc., Chicago, IL, USA). A p<0.05 was considered statistically significant.
The baseline characteristics of the subjects are shown in Table 1. No significant differences existed between the two treatment groups. There were 48 men and 5 women; the patients' mean age was 60.7±6.8 years. Fourteen of the patients had type 2 diabetes. None of the patients experienced any drug-related complications during the 8 weeks of treatment.
Both groups showed significant improvements in systolic blood pressure (control group, from 153.4±14.7 mmHg to 137.9±14.3 mmHg; rosuvastatin group: from 154.4±14.3 mmHg to 132.8±13.8 mmHg; p<0.01) and FMD (control group, from 7.5±3.1% to 9.9±2.9%; rosuvastatin group, from 7.8±3.5% to 10.5±3.6%; p<0.01) after 8 weeks compared with baseline. However, there were no significant differences between the two groups after 8 weeks of treatment (Table 2). The control group did not show significant changes in the lipid profile, but the rosuvastatin group showed improvement in total cholesterol (from 218.2±36.9 mg/dl to 167.1±43.0 mg/dl; p<0.01), LDL-cholesterol (from 147.5±33.3 mg/dl to 101.8±32.4 mg/dl; p<0.01), and triglycerides (from 174.0±61.9 mg/dl to 136.8±64.6 mg/dl; p<0.01; Table 2). Neither group showed a significant change in the high-sensitivity C-reactive protein level from baseline to 8 weeks.
There were no significant differences in fasting glucose, fasting insulin, QUICKI, HOMA, or adiponectin levels between the two groups before or after randomization (Table 2). The mean delta changes in HbA1c (3.0±10.1% vs. -1.3±12.7%; p=0.33), fasting glucose (-1.3±18.0% vs. 2.5±24.1%; p=0.69), and fasting insulin levels (5.2±70.5% vs. 22.6±133.2%; p=0.27) in the control and rosuvastatin treatment groups were not significantly different (Fig. 2). Furthermore, the mean delta changes of the QUICKI (2.2±11.6% vs. 3.6±11.9%; p=0.64) and HOMA index (11.6±94.9% vs. 32.4±176.7%; p=0.44) also were not significantly different between the control and rosuvastatin groups (Fig. 3). The plasma adiponectin level increased significantly in both groups compared with baseline. However, there was no significant difference in the mean delta change between the control and rosuvastatin groups (23.2±28.4% vs. 23.1±27.6%; p=0.36; Fig. 4).
The current study showed that 8 weeks of rosuvastatin (20 mg daily) therapy resulted in no significant improvement or deterioration in fasting glucose levels, adiponectin levels, or insulin resistance. As expected, all components of the lipid profile improved more from baseline following rosuvastatin treatment than control treatment. Our results suggest that rosuvastatin did not cause glucose intolerance or insulin resistance.
Insulin resistance is associated with increased risk for CVD.15,16 The association between insulin resistance and hypertension is controversial. Whereas some studies have reported that insulin resistance is strongly related to hypertension, others have shown only a weak or even no association.17-19 In clinical practice, risk factors for CVD tend to cluster within individuals, and hypertensive patients are at increased risk for metabolic syndrome and adverse changes in insulin resistance and the lipid profile. For risk modification, statins are prescribed in patients with multiple risk factors for CVD.
Recent clinical studies have demonstrated that lipophilic statins, such as atorvastatin, simvastatin, and the hydrophilic statin rosuvastatin might increase the onset of new diabetes.3,9,10 However, these studies were not designed to evaluate the onset of new diabetes or insulin resistance. Therefore, these results are not clear and have not led to recommendations for the general population. Other researchers have previously reported that simvastatin reduces adiponectin levels and insulin sensitivity.20 Previously, Koh et al.11 published that atorvastatin treatment in healthy hyperlipidemic patients aggravates insulin resistance by increasing fasting glucose, insulin, and HbA1c levels at relatively high doses. The characteristics of the patients in both studies were similar. The baseline characteristics, such as lipid level, proportion of diabetic patients, and laboratory findings of baseline insulin resistance were similar, even though the patient group in that study was composed of healthy volunteers and our patient group consisted of newly diagnosed hypertensive, dyslipidemic patients.11 Indeed, whether statins, especially atorvastatin, have a decisive effect on insulin resistance is unclear. Recently, Koh et al.21 published that compared with pravastatin, rosuvastatin therapy significantly increased fasting insulin and HbA1c while decreasing plasma adiponectin levels and the QUICKI index compared with baseline. A reason may exist for this discordance. First, our patients simultaneously took telmisartan 80 mg, which has a PPAR-γ effect that improves insulin resistance. As a result, it follows that it may have had some masking effects. This is a limitation of our study protocol. Second, our study groups consisted of hypertensive, dyslipidemic patients and included some patients with diabetes. Our patients already had metabolic disease. Thus, the unwanted metabolic effect by rosuvastatin may have been relatively weaker than in the patients in Koh et al.'s study.
Huptas et al.6 showed that 6 weeks of atorvastatin treatment results in significant improvement in insulin sensitivity in patients with metabolic syndrome. But, these conflicting results cannot be explained. Furthermore, it is unknown whether different statins have different metabolic effects on the basis of their lipophilic properties. Similar findings were shown for pravastatin, which is nonlipophilic.22,23 Another study compared the effects of atorvastatin (10 mg) and rosuvastatin (10 mg) on changes in glucose and insulin levels, and the HOMA of the insulin resistance index, which were not significantly different between the two groups.24 Also, the result of a meta-analysis of randomized controlled trials may suggest that potential differences exist between statins.25 It is not clear why various statins have beneficial metabolic actions in some studies, but not in others. Thus, further head-to-head comparative studies are needed to elucidate the effects of statins on glucose metabolism.
Our results showed that lipid levels improved, adiponectin levels increased, and the percentage change in fasting glucose and insulin levels and the QUICKI and HOMA indexes were not significantly different between the rosuvastatin and control treatment groups. To determine the trends in each group's differences according to treatment, we assessed the mean value of each parameter and the mean of the delta change. The values shown in Table 2 and the mean change percentages (Fig. 2-4) for each parameter may seem to be different results. But this could be because of the statistical differences. Studies in an animal model of insulin resistance suggested that rosuvastatin treatment increases whole-body and peripheral tissue insulin sensitivity via improved cellular insulin signal transduction.26 A 20 mg dose of rosuvastatin, which is a relatively high dose, was used in our study. Rosuvastatin (20 mg) has equal lipid-lowering potency as atorvastatin (40 mg). Therefore, we assume that each statin has differential effects on insulin sensitivity and the rate of new-onset diabetes according to dosage.
The rosuvastatin (20 mg) group tended to show improved vascular endothelial function and FMD, but showed no significant difference at the time of study termination. Our study and another study showed that treatment with a statin improved FMD in patients with a decreased baseline FMD.27 In that study, discontinuation of statin treatment reversed the improved FMD to baseline.27 The results showed that statins definitely affect vascular endothelial function, but only in patients with increased cardiovascular disease risk factors. In the current study, most patients had low cardiovascular disease risk factors; the anti-hypertensive ARB therapy could have already resulted in maximum improvement of vascular endothelial function. Under such conditions, statins would not have an additional effect on vascular endothelial function owing to the ceiling effect. If the current study had enrolled more patients with diabetes, metabolic syndrome, or other cardiovascular disease, the results would possibly have greater meaning.
In our data, the value of adiponectin increased in both groups but did not differ significantly between the two groups. Some diabetic patients were included in this study, because many hypertensive patients already show metabolic disease in the real world. As a natural consequence, it follows that analysis of our data was partially ambiguous. Furthermore, telmisartan 80 mg, which has a PPAR-γ effect that improves insulin resistance, was taken by all patients for adequate BP control. As a result, it follows that the ARB may have shown good BP control but some masking effects on adiponectin, inflammatory markers, and insulin resistance.
In conclusion, our study showed that 8 weeks of rosuvastatin (20 mg daily) therapy showed no significant improvement or deterioration of fasting glucose levels, insulin resistance, and adiponectin levels in newly diagnosed hypertensive patients treated with the ARB telmisartan.
Figures and Tables
Fig. 2
Percentage change in HbA1C, fasting glucose, and fasting insulin levels. The control and rosuvastatin treatment groups did not show significant changes in HbA1C levels (mean change, 3.0±10.1% vs. -1.3±12.7%; p=0.33), fasting glucose levels (-1.3±18.0% vs. 2.5±24.1%; p=0.69), or fasting insulin levels (mean change, 5.2±70.5% vs. 22.6±133.2%; p=0.27) from baseline.

Fig. 3
Percentage change in QUICKI and HOMA indices. The control and rosuvastatin treatment groups did not show significant changes in the QUICKI index (mean change, 2.2±11.6% vs. 3.6±11.9%; p=0.64) or the HOMA index (11.6±94.9% vs. 32.4±176.7%; p=0.44). QUICKI: Quantitative Insulin-Sensitivity Check Index, HOMA: Homeostasis Model Assessment.

Fig. 4
Percentage change in adiponectin level. The adiponectin level significantly increased in the rosuvastatin group (p=0.046) but showed no significant difference compared with the control group (mean change, 23.2±28.4% vs. 23.1±27.6%; p=0.36).

TABLE 2
Comparison of lipid and endocrine parameters between the control and rosuvastatin groups

*p<0.05 comparison with each baseline value. HDL: high-density lipoprotein, LDL: low-density lipoprotein, hs-CRP: high-sensitivity C-reactive protein, HbA1C: glycated hemoglobin, QUICKI: quantitative insulin-sensitivity check index, HOMA: homeostasis model assessment, BP: blood pressure, FMD: flow-mediated vasodilation.
ACKNOWLEDGEMENTS
This research was supported by the Bio R&D program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2010-0019913).
References
1. Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007. 18:58–65.

2. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999. 21:1009–1060.
3. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008. 359:2195–2207.

4. Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000. 47:648–657.

5. Koh KK, Quon MJ, Han SH, Ahn JY, Jin DK, Kim HS, et al. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005. 45:1088–1093.

6. Huptas S, Geiss HC, Otto C, Parhofer KG. Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. Am J Cardiol. 2006. 98:66–69.

7. Takarada S, Imanishi T, Ishibashi K, Tanimoto T, Komukai K, Ino Y, et al. The effect of lipid and inflammatory profiles on the morphological changes of lipid-rich plaques in patients with non-ST-segment elevated acute coronary syndrome: follow-up study by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv. 2010. 3:766–772.

8. Katsiki N, Tziomalos K, Chatzizisis Y, Elisaf M, Hatzitolios AI. Effect of HMG-CoA reductase inhibitors on vascular cell apoptosis: beneficial or detrimental? Atherosclerosis. 2010. 211:9–14.

9. Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003. 361:2005–2016.

10. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. ASCOT investigators. Preventionof coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003. 361:1149–1158.

11. Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010. 55:1209–1216.

12. Devaraj S, Siegel D, Jialal I. Simvastatin (40 mg/day), adiponectin levels, and insulin sensitivity in subjects with the metabolic syndrome. Am J Cardiol. 2007. 100:1397–1399.

13. Kim W, Jeong MH, Cho SH, Yun JH, Chae HJ, Ahn YK, et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circ J. 2006. 70:1052–1057.

14. Park CS, Kim W, Woo JS, Ha SJ, Kang WY, Hwang SH, et al. Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int J Cardiol. 2010. 145:261–262.

15. Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Osaka CAD Study Group. Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003. 23:85–89.
16. Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004. 109:551–556.
17. Saad MF, Rewers M, Selby J, Howard G, Jinagouda S, Fahmi S, et al. Insulin resistance and hypertension: the Insulin Resistance Atherosclerosis study. Hypertension. 2004. 43:1324–1331.
18. Diabetes Prevention Program Research Group. Hypertension, insulin, and proinsulin in participants with impaired glucose tolerance. Hypertension. 2002. 40:679–686.
19. Mitchell BD, Haffner SM, Hazuda HP, Valdez R, Stern MP. The relation between serum insulin levels and 8-year changes in lipid, lipoprotein, and blood pressure levels. Am J Epidemiol. 1992. 136:12–22.

20. Koh KK, Quon MJ, Han SH, Lee Y, Ahn JY, Kim SJ, et al. Simvastatin improves flow-mediated dilation but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care. 2008. 31:776–782.

21. Koh KK, Quon MJ, Sakuma I, Han SH, Choi H, Lee K, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2011. [Epub ahead of print].

22. Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, et al. LIPID Study Group. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. 2003. 26:2713–2721.

23. Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001. 103:357–362.

24. Park JS, Kim YJ, Choi JY, Kim YN, Hong TJ, Kim DS, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010. 25:27–35.

25. Coleman CI, Reinhart K, Kluger J, White CM. The effect of statins on the development of new-onset type 2 diabetes: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008. 24:1359–1362.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download