Abstract
The aim of this study was to compare the efficacy, rebleeding rates, survival, and complications of endoscopic variceal ligation (EVL) with those of endoscopic variceal obliteration (EVO) in patients with acute type 1 gastroesophageal variceal (GOV1) bleeding. Data were collected retrospectively at a single center. A total of 84 patients were selected (20 patients underwent EVL; 64 patients underwent EVO) from February 2004 to September 2011. Their clinical characteristics, laboratory results, vital signs, Child-Pugh score, Model for End-stage Liver Disease (MELD) score, and overall mortality were evaluated. There were no significant differences in baseline characteristics between the two groups. The success rate in initial control of active bleeding was not significantly different between the EVL and EVO groups (18/20 EVL, or 90.0%, compared with 62/64 EVO, or 96.9%; p=0.239). The early rebleeding rate was also not significantly different between the groups (3/18 EVL, or 16.7% compared with 17/62 EVO, or 27.4%; p=0.422). The late rebleeding rate of the EVL group was lower than that of the EVO group (3/18 EVL, or 16.7%, compared with 26/59 EVO, or 44.1%; p=0.042). The time-to-rebleeding was 594 days for the EVL group and 326 days for the EVO group (p=0.054). In the multivariate analysis, portal vein thrombosis (PVT) was a significant risk factor for early rebleeding. Hepatocellular carcinoma (HCC) and previous history of bleeding were significant risk factors for very late rebleeding. In conclusion, EVL is better than EVO in reducing late rebleeding in acute GOV1 bleeding. HCC, PVT, and previous bleeding history were significant risk factors for rebleeding.
Gastric variceal bleeding is an important cause of gastrointestinal bleeding in patients with portal hypertension. Gastric varices occur in approximately 20% of patients with portal hypertension.1,2 Gastric variceal bleeding is less common than esophageal variceal bleeding, but bleeding from gastric varices is more severe and more difficult to treat. Furthermore, gastric variceal bleeding results in higher morbidity and mortality than esophageal variceal bleeding.3,4
Because endoscopic variceal ligation (EVL) and endoscopic variceal obliteration (EVO) are readily available and inexpensive, these procedures have been successfully used in many centers for the treatment of acute gastric variceal bleeding.5,6 In type 2 gastroesophageal variceal (GOV2) bleeding, EVO is the most effective therapy, whereas in GOV1 bleeding, either EVL or EVO can be used.7,8 Although endoscopic variceal band ligation is the undisputed gold standard therapy for bleeding esophageal varices, this approach has been less successful for the treatment of bleeding gastric varices.5 Currently, among the endoscopic therapeutic options for gastric variceal bleeding, the greatest evidence for successful treatment exists for EVO using N-butyl-2-cyanoacrylate, which is recommended as a first-line endoscopic therapy.9-11 However, the optimal management of bleeding gastric varices remains an open question owing to the lack of large randomized controlled trials. Uncontrolled data comparing therapies in bleeding fundal varices showed that the best control of initial hemorrhage (90-100%) is achieved with EVO, transjugular intrahepatic portosystemic shunt, or ballooned retrograde transvenous obliteration.12 In acute GOV1 bleeding, however, there are limited data comparing EVL and EVO. Three small single-center randomized controlled trials compared EVO versus endoscopic sclerotherapy13 or band ligation in bleeding gastric varices.6 All three trials were favorable for EVO regarding control of acute hemorrhage,5,13 rebleeding, or complication rates.6
Lo et al.5 reported that EVL is less effective and more difficult than EVO in the management of gastric variceal bleeding. In that study, rebleeding rates were significantly higher in the EVL group (54%) than in the EVO group (31%).5 However, another study showed no significant difference in survival between the two groups.6
Currently, controversies exist and data are limited on the management of acute GOV1 bleeding. Therefore, the present study aimed to compare the efficacy of EVL and EVO in patients with acute GOV1 bleeding and to analyze the clinical risk factors for rebleeding, complications, and overall mortality.
From February 2004 to September 2011, 298 patients with gastric variceal bleeding who visited Chonnam National University Hospital were selected. Among them, 196 patients with esophageal variceal bleeding or a previous history of variceal bleeding were excluded. Eighteen patients were excluded owing to other systemic comorbidities (10 had hepatorenal syndrome, 3 were on dialysis, 3 had multiple metastatic malignancy, 1 had acute respiratory failure, and 1 had concomitant ulcer bleeding). Eighty-four patients with acute GOV1 bleeding were selected. The medical records of all identified patients were reviewed to collect data on clinical characteristics, outcomes, and follow-up. In the case of multiple hospital visits, only the initial admission was considered to avoid bias. For patients with multiple bleeding events during the study period, only the initial event was considered. Initial general managements were done in every patient. Hemodynamic abnormalities were corrected, vasoactive drugs (terlipressin, octreotide) were used, and antibiotic prophylaxis was performed for the prevention of complications. All subjects underwent emergency EVL or EVO within 12 hours of presentation to the emergency department. Eighty-four patients were selected for study (20 patients underwent EVL; 64 underwent EVO). Their clinical characteristics, laboratory results, vital signs, endoscopic findings, Child-Pugh score, Model for End-stage Liver Disease (MELD) score, and overall mortality were evaluated.
The cause of liver cirrhosis (alcohol, hepatitis B virus, hepatitis C virus), the presence of underlying hepatocellular carcinoma (HCC), the presence of portal vein thrombosis (PVT) on an abdominal CT, and previous variceal bleeding history were reviewed for all patients. During emergency endoscopy, EVL was performed by occluding the protruding variceal column with elastic rubber rings by using a transparent cap attached to the distal end of the endoscope. Commercially available sclerotherapy injectors were used for EVO. N-Butyl-2-cyanoacrylate (Histoacryl®; B. Braun Dexon, Spangenberg, Germany) was mixed with ethiodized oil (Lipiodol®; Guerbert, Roissy, France) and was injected as a bolus dose of 0.5 to 2 ml, depending on the size of the gastric varices. In general, injections were directly delivered into gastric varices with active bleeding or containing stigmata of recent bleeding.
On the basis of the endoscopy report, varices were classified by using the description provided by Sarin et al.1: type 1 (GOV1) varices are continuous with esophageal varices and extending along the lesser curvature of stomach for 2 to 5 cm below the gastroesophageal junction, and type 2 (GOV2) varices extend from the esophagus below the gastroesophageal junction toward the fundus. The form of gastric or esophageal varices was classified into three types: F1, small and tortuous; F2, medium-sized and nodular; and F3, large and tumorous.14
Bleeding was considered to arise from gastric varices if one of the following lesions was present: spurting, oozing, white nipple sign, erosion, or adherent blood clot.15,16 Initial hemostasis was defined by the presence of stable vital signs and the absence of rebleeding from follow-up endoscopy. Early rebleeding was defined as occurring within 6 weeks, late rebleeding within 6 weeks to 1 year, and very late rebleeding after 1 year of initial hemostasis.17
Analysis was performed by using SPSS 20.0.0 (SPSS, Inc, an IBM Company, Chicago, IL, USA). In the event of missing data values, data were not replaced. Data were expressed as percentages, means±SD, or medians and ranges as appropriate. Mann-Whitney U-test was used to analyze continuous variables between the groups. Fisher's exact test was used for categorical data. Univariate and multivariate survival analyses were performed on selected variables, including age, presence of HCC, presence of PVT, previous bleeding history, etiology (ALD vs non-ALD), MELD score (≥13 vs <13), presence of infection, and gastric varices size (F2/F3 vs F1). Kaplan-Meier analysis was used to analyze overall survival and time to rebleeding. Null hypotheses of no significant difference were rejected if p values were less than 0.05.
The median age of the 84 patients was 57.0 years (range, 52.0-64.1 years) and there was a male predominance in gender (73 males, 86.9%). The cause of cirrhosis was alcoholic liver disease (n=38, 45.2%), viral hepatitis B (n=37, 44.0%), viral hepatitis C (n=7, 8.3%), and unknown etiologies (n=2, 2.4%). The size of the gastric varices were mostly F2 (n=36, 42.9%) and F3 (n=36, 42.9%), with 12 cases of F1 (14.3%). Nineteen patients (22.6%) had coexisting HCC, 28 (33.3%) patients had PVT, and 49 (58.3%) patients had a previous history of variceal bleeding.
The analysis included 20 patients in the EVL group and 64 patients in the EVO group. Baseline clinical characteristics were not significantly different between the two groups (Table 1).
Possible complications of endoscopic treatment were detected in both groups. In the EVL group, minor complications such as mild fever (1/20, 5.0%) and EVL-induced ulcer (1/20, 5.0%) and major complications such as aspiration pneumonia (1/20, 5.0%) were noted. In the EVO group, minor complications such as mild fever (1/64, 1.5%) and EVO-induced ulcer (1/64, 1.5%) and major complications such as aspiration pneumonia (5/64, 7.8%) and embolism (1/64, 1.5%) (e.g., splenic infarction) were noted. A notable finding was that the EVO group had more severe complications: infections and splenic infarction. The EVL group had fewer complications, although this was not statistically significant (p=0.635).
Success rates in the initial control of active bleeding were not significantly different between the EVL and EVO groups [90.0% (18/20) vs. 96.9% (62/64); p=0.239]. Only one patient in the EVO group failed to achieve hemostasis within 48 hours.
The early rebleeding rates (within 6 weeks) of the EVL and EVO groups were 16.7% and 27.4% (3/18 vs. 17/62; p=0.422). However, the late rebleeding rate of the EVL group was lower than that of the EVO group: 16.7% and 44.1%, respectively (3/18 vs. 26/59; p=0.042). The very late rebleeding rates (over 1 year) of the EVL and EVO groups were similar: 64.7% and 62.0%, respectively (11/17 vs. 31/50; p=0.634). In conclusion, EVL was associated with a lower rate of late rebleeding (Table 2).
Although not statistically significant, the median time to rebleeding was longer in the EVL group than in the EVO group (594 days vs. 326 days, p=0.054). The cumulative rate of rebleeding, computed by Kaplan-Meier survival analysis, showed no significant difference between the EVL and EVO groups. However, the EVL group demonstrated a positive trend toward a longer event-free period (EVL: 489±319 vs. EVO: 175±63 p=0.067) As shown in Fig. 1, the time to rebleeding was longer in the EVL group.
In the univariate analysis for early rebleeding, the presence of HCC (OR: 4.418, 95% CI: 1.457-13.402, p=0.009) and PVT (OR: 4.500, 95% CI: 1.561-12.969, p=0.005) were independent predictive factors (Table 3). In the multivariate analysis, PVT was a risk factor for early rebleeding (OR: 4.500, 95% CI: 1.561-12.969, p=0.005) (Table 3). In the univariate analysis for very late rebleeding, the presence of HCC (OR: 5.278, 95% CI: 1.577-17.668, p=0.007), PVT (OR: 3.029, 95% CI: 1.165-7.873, p=0.023), and previous bleeding history (OR: 3.026, 95% CI: 1.225-7.474) were independent predictive factors (Table 4). In the multivariate analysis, the risk factors for very late rebleeding were HCC (OR: 5.06, 95% CI: 1.464-17.495, p=0.01) and previous bleeding history (OR: 2.90, 95% CI: 1.124-7.488, p=0.028) (Table 4). The statistical analysis of late rebleeding showed no significant differences between the clinical factors (results not shown).
Thirteen patients died within 1 year after initial endoscopic hemostasis. The 1-year mortality rate of the EVL and EVO groups was 5.6% and 19.4% (1/18 vs. 12/62, p=0.138). The median survival of the EVL and EVO groups was 1160 days and 852 days (p=0.115). The cumulative survival rate, computed by Kaplan-Meier survival analysis, showed no significant difference between the EVL and EVO groups (Fig. 2).
Compared with esophageal varices, the treatment methods for bleeding gastric varices are less well established. Traditional endoscopic therapies used for esophageal varices, such as band ligation and injection of sclerosing agents, have also been trialed in gastric varices with limited success with respect to hemostasis, rebleeding, and obliteration of gastric varices.5,13
This study showed that both EVL and EVO were effective for controlling the majority of GOV1-related bleeding. However, EVL was associated with a lower rate of late rebleeding and there was a trend toward longer survival and time-to-rebleeding. It is well known that esophageal varix band ligation is more effective than esophageal varix obliteration in esophageal varices. Because GOV1 constitutes an extension of esophageal varices along the lesser curvature of the stomach, EVL may be more effective in GOV1 bleeding.7,18
According to some previous studies, EVL had complications including transient dysphagia, chest discomfort, superficial ulcers at the banding site, bacteremia, and infection.19 EVO had minor complications such as chest pain, pleural effusion, dysphagia, and fever. Severe complications include deep ulcers through the gastric wall which predispose to hemorrhage, stricture formation, perforation, and embolism due to the sclerosing agent.8 In this study, the EVO group had more severe complications such as infection and splenic infarction, whereas the EVL group had fewer complications. There were some case reports of pulmonary embolism, cerebral embolism, and other complications after N-butyl-2-cyanoacrylate injection.20,21
The risk of late rebleeding (more than 6 weeks after the initial episode) is known to be related to factors such as continued alcohol consumption, variceal size, renal failure, degree of liver failure, and presence of HCC.22 However, there were no statistically significant risk factors for late rebleeding in our study; this might have been due to the limitation in the number of patients. PVT was the only significant risk factor for early rebleeding in this study, and the risk factors for very late rebleeding were HCC and previous bleeding history. In a similar study, Tan et al.6 reported that there were no significant differences in survival but the rebleeding rate was lower in the EVO group, which is in contrast with our study. Tan et al.6 included both GOV1 and GOV2 in their study, whereas we included only GOV1. This might have caused the difference between the two studies. At present, current guidelines suggest that either EVL or EVO is recommended for the treatment of GOV1 bleeding.7 In this study, however, EVL had better clinical outcomes and lower complications in GOV1 bleeding. Because there is no definite guideline in the treatment of GOV1 bleeding, most doctors prefer EVO to EVL because it is readily available and easy to approach, especially in large varices (>2 cm). However, our study showed that EVL has advantages in rebleeding risk and post-procedural complications. Therefore, it could be suggested as a primary treatment option in GOV1 bleeding.
There are limitations to this study. Retrospective research is potentially susceptible to several biases. The experience of a single center may not be applicable generally. The rate of PVT was higher in the EVL group, whereas the rate of HCC was higher in the EVO group. The discordance of the rate of such factors could have contributed to the difference in prognosis. The medical records of some of the patients were lacking accurate history about underlying disease and medication history (especially beta-blockers). The method of bleeding management between EVL and EVO was selected by several doctors without protocols. Also, endoscopic findings were reviewed by medical records, which were described by several doctors. Doctors preferred EVO to EVL in our center because of the previously described reasons. This resulted in an imbalance in the number of cases, which could have caused a selection bias.
In conclusion, EVL is a more effective treatment modality than EVO in the management of acute GOV1 bleeding. The presence of PVT, HCC, and previous variceal bleeding history are independent risk factors for rebleeding.
Figures and Tables
FIG. 1
Cumulative risk of rebleeding of both treatment modalities (EVL vs. EVO) by use of Kaplan-Meier curve with log-rank test. The EVL group demonstrated a positive trend toward a longer event-free period.

FIG. 2
Cumulative survival rate of both treatment modalities (EVL vs. EVO) by use of Kaplan-Meier curve with log-rank test. The EVL group demonstrated a positive trend toward a longer survival rate.
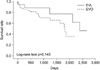
TABLE 1
Baseline demographics of patients with GOV1 bleeding

Data are presented as medians and interquartile ranges (25th and 75th percentiles) for continuous variables and as numbers (percentages) for categorical variables. GOV: gastroesophageal variceal, EVL: endoscopic variceal ligation, EVO: endoscopic variceal obliteration, HBV: hepatitis B virus, HCV: hepatitis C virus, BP: blood pressure, PT: prothrombin time, MELD: model for end-stage liver disease, GV: gastric varices.
References
1. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992. 16:1343–1349.

2. Thakeb F, Salem SA, Abdallah M, el Batanouny M. Endoscopic diagnosis of gastric varices. Endoscopy. 1994. 26:287–291.

3. Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988. 95:434–440.

4. Arakawa M, Masuzaki T, Okuda K. Pathomorphology of esophageal and gastric varices. Semin Liver Dis. 2002. 22:73–82.

5. Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001. 33:1060–1064.

6. Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006. 43:690–697.

7. Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, et al. Korean Association for the Study of the Liver. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012. 18:1–21.

8. Peck-Radosavljevic M, Trauner M, Schreiber F; Austrian Society of Gastroenterology and Hepatology. Austrian consensus on the definition and treatment of portal hypertension and its complications. Endoscopy. 2005. 37:667–673.

9. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases. Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007. 46:922–938.

10. Qureshi W, Adler DG, Davila R, Egan J, Hirota W, Leighton J, et al. Standards of Practice Committee. ASGE Guideline: the role of endoscopy in the management of variceal hemorrhage, updated July 2005. Gastrointest Endosc. 2005. 62:651–655.

11. de Franchis R. Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010. 53:762–768.

12. Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004. 126:1175–1189.

13. Sarin SK, Jain AK, Jain M, Gupta R. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol. 2002. 97:1010–1015.

14. Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981. 27:213–218.

15. Ramond MJ, Valla D, Mosnier JF, Degott C, Bernuau J, Rueff B, et al. Successful endoscopic obturation of gastric varices with butyl cyanoacrylate. Hepatology. 1989. 10:488–493.

16. Hou MC, Lin HC, Kuo BI, Lee FY, Schmidt CM, Lee SD. Clinical implications of the white nipple sign and its role in the diagnosis of esophageal variceal hemorrhage. Am J Gastroenterol. 1996. 91:2103–2109.
17. Jun CH, Park CH, Lee WS, Joo YE, Kim HS, Choi SK, et al. Antibiotic prophylaxis using third generation cephalosporins can reduce the risk of early rebleeding in the first acute gastroesophageal variceal hemorrhage: a prospective randomized study. J Korean Med Sci. 2006. 21:883–890.

18. Toubia N, Sanyal AJ. Portal hypertension and variceal hemorrhage. Med Clin North Am. 2008. 92:551–574.

19. Garcia-Pagán JC, Bosch J. Endoscopic band ligation in the treatment of portal hypertension. Nat Clin Pract Gastroenterol Hepatol. 2005. 2:526–535.

20. Mahadeva S, Bellamy MC, Kessel D, Davies MH, Millson CE. Cost-effectiveness of N-butyl-2-cyanoacrylate (histoacryl) glue injections versus transjugular intrahepatic portosystemic shunt in the management of acute gastric variceal bleeding. Am J Gastroenterol. 2003. 98:2688–2693.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download