Abstract
Drug-eluting stent implantation is an effective treatment for coronary artery disease, yet unexpected serious complications during stent implantation are possible. A 70-year-old man with unstable angina presented with a left main bifurcation lesion. Two drug-eluting stents were successfully deployed at the left main bifurcation lesion by the mini-crush technique under intravascular ultrasound guidance. However, after removal of the wire and intravascular ultrasound catheter, the stent of the proximal left circumflex artery was damaged and shortened at the distal edge. We used a looping wire technique to cross the injured stent and we successfully re-dilated the damaged portion of the stent. Finally, we deployed an additional drug-eluting stent at the left circumflex artery over the damaged stent. Our case illustrates the importance of gentle handling of devices during coronary intervention. Furthermore, interventionists should keep in mind the role of intravascular ultrasound when treating this kind of serious complication.
Drug-eluting stent (DES) implantation is an effective treatment for coronary artery disease, yet adverse events are possible owing to unexpected serious complications during DES implantation. However, stent crushing injury is a rare complication. We describe here a looping wire technique to cross a damaged stent with subsequent additional DES implantation over the damaged stent in the left circumflex artery.
A 70-year-old man with unstable angina presented to our emergency room. He had a history of essential hypertension. Sixty-six months previously, he had been admitted owing to unstable angina pectoris and two coronary stents had been implanted at the first obtuse marginal (OM) branch (2.5×20 mm Taxus®, Boston Scientific, Natick, MA) and the proximal left anterior descending artery (LAD) (3.0×33 mm Cypher®, Cordis Corporation, Miami Lakes, FL), respectively.
Diagnostic coronary angiography showed significant stenosis (90% diameter stenosis) at the proximal left circumflex (LCX) and moderate stenosis at the proximal LAD with distal left main involvement. We deployed two everolimus-eluting stents (EESs) at both the proximal LCX (3.5×23 mm Xience™ V stent, Abbott Vascular, Abbott Park, IL) and the distal left main/proximal LAD (4.0×28 mm Xience™ V stent) by use of the mini-crush technique under intravascular ultrasound (IVUS) guidance (iLab® Ultrasound Imaging System, Boston Scientific, Minneapolis, MN). We did final kissing balloon angioplasty and the IVUS showed good apposition and expansion of the two EESs. Coronary angiography showed good left coronary flow and no residual stenosis. However, after removal of the IVUS catheter, there was distal edge damage and shortening of the EES at the LCX (Fig. 1). Because of the patient's consent for the percutaneous treatment, we tried to cross the injured stent lumen with many kinds of wires, but this was ineffective. Therefore, we used a looping wire technique with a 0.014'' hi-torque Balance Middleweight (BMW) universal coronary guide wire (Abbott Vascular) to cross the injured stent and we successfully re-dilated the damaged portion of the stent by using multiple balloons [Ryujin® 1.25 mm (Terumo, Somerset, NJ), Voyager® 2.5 mm (Abbott Vascular), Voyager® 3.5 mm (Abbott Vascular) (Fig. 2)]. Finally, we deployed an additional EES (3.5×18 mm Xience™ V stent, Abbott Vascular) at the LCX over the injured stent. Coronary angiography showed good flow with dye staining at the LCX (Fig. 2). IVUS using the pull-back system from the distal coronary artery showed good expansion and the three EESs were well apposed, except for the partially crushed first-deployed EES of the proximal LCX (Fig. 3). There was no recurrence of chest pain and the patient was discharged from our hospital after intensive medication. The patient has been well during 3 years of clinical follow-up.
Percutaneous coronary intervention (PCI) can be performed safely and effectively owing to improvements in different medical technologies. However, high-risk patients with difficult coronary anatomy and high-risk clinical characteristics can experience adverse clinical outcomes. Even though the overall incidence of acute complications by PCI has decreased over the years, these complications have not been eliminated.1,2 The early complications after DES implantation included cardiac death, stroke, perforation, stent thrombosis due to various reasons, renal failure, retroperitoneal bleeding, and vascular complications. Late complications related to DESs include stent fracture, restenosis, aneurysm formation, and late stent thrombosis.3-6
We performed the mini-crush technique over the left main bifurcation lesion with final kissing balloon angioplasty. However, the present case showed a serious complication after the removal of the IVUS catheter. Possible explanations for the damage at the distal edge of the LCX stent were both IVUS and guiding catheter damage to the LCX stent. IVUS catheter injury or entrapment during PCI sometimes occurs as the result of mal-apposed stent struts, catheter deformation by multiple uses, or inadequate wire positioning in the tortuous segment of the vessel.7,8 Rewiring to the LCX after mini-crushing stenting and removal of the original guide wire of the LCX might have resulted in inadequate wire positioning in our case. If the IVUS catheter is advanced through the stent, then entrapment of the IVUS catheter can occur. Because we used a new IVUS catheter in our case, entrapment of both the IVUS and guiding catheters by the tortuous vessel or inadequate wire positioning over the left main bifurcation lesion might have been the cause of stent damage. Several cases of pressure wire kinking by IVUS catheter or stent crushing by IVUS entrapment have been reported.9,10 These cases were resolved by the application of torque along the catheter with gentle forward movement over the wire or the passage of a new wire across the lateral side with sequential balloon dilatation, respectively.
Because of the risk of stent thrombosis, the gold standard of treatment in our case would be surgical correction. However, we deployed an additional stent over the damaged stent by the percutaneous method according to the patient's preference. Because of possible target lesion failure after balloon angioplasty without stent implantation, we deployed an additional stent. The looping wire technique entails making a loop of wire before the entrance of the crushed stent strut and gentle pushing toward the distal portion. It is possible to avoid the entrance of the false position by an increased cross-sectional diameter at the tip of the looping wire. Although our case was not unique in terms of the looping wire technique, it is very rare to use this technique in cases of crushed and shortened stents.
The clinical significances of our case are as follows: 1) gentle handling of the IVUS and guiding catheters after PCI is important, 2) mechanical complication of a damaged stent is possible and careful interpretation of both the coronary angiogram and the IVUS image are important, 3) rewiring using a looping wire technique could be a percutaneous treatment option by avoiding a false wire position, and a new wire that crosses the lesion should be confirmed by IVUS immediately after wiring or after small-sized balloon dilatation.
In conclusion, our case demonstrated a possible option for the percutaneous treatment of a crushed stent and the value of IVUS examination in both diagnosis and treatment.
Figures and Tables
FIG. 1
Coronary angiography was done after implantation of two stents. After removal of the IVUS catheter, there was distal edge damage and shortening of the EES at the LCX in both the RAO caudal (A, black arrow) and spider view (B). IVUS: intravascular ultrasound, EES: everolimus-eluting stent, LCX: left circumflex artery, RAO: right anterior oblique.
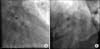
FIG. 2
We performed a looping wire technique using a 0.014'' hi-torque Balance Middleweight universal coronary guide wire (Abbott Vascular, Santa Clara, CA, USA) to cross the damaged stent and we successfully re-dilated the damaged portion (A). The final coronary angiography showed good flow with dye staining at the LCX (B). LCX: left circumflex artery.

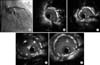
FIG. 3
Coronary angiography after EES implantation (A): IVUS finding in each point. IVUS using a pull-back system from the distal coronary artery showed good expansion and the three well-apposed EESs at the distal left main (E), the bifurcation of the left main (D), and the proximal LCX (C), with the exception of the partially crushed EES (B, white arrow). IVUS: intravascular ultrasound, EES: everolimus-eluting stent, LCX: left circumflex artery.
References
1. Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, et al. Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000; 102:2945–2951.

2. Holmes DR, Selzer F, Johnston JM, Kelsey SF, Holubkov R, Cohen HA, et al. National Heart, Lung, and Blood Institute Dynamic Registry. Modeling and risk prediction in the current era of interventional cardiology: a report from the National Heart, Lung, and Blood Institute Dynamic Registry. Circulation. 2003; 107:1871–1876.

3. Lee SR, Jeong MH, Ahn YK, Chae SC, Hur SH, Kim YJ, et al. Korea Acute Myocardial Infarction Registry Investigators. Clinical safety of drug-eluting stents in the Korea acute myocardial infarction registry. Circ J. 2008; 72:392–398.

4. Lee KH, Lee SR, Jin GY, Lee SH, Rhee KS, Chae JK, et al. Double coronary artery stent fracture with coronary artery microaneurysms. Int Heart J. 2009; 50:127–132.

5. Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007; 69:387–394.

6. Fang HY, Bhasin A, Youssef A, Hsueh SK, Fang CY. Intravascular ultrasound (IVUS) guided fixation of an accidentally crushed coronary stent. Int Heart J. 2008; 49:621–627.

7. Hong YM, Lee SR. A case of guide wire fracture with remnant filaments in the left anterior descending coronary artery and aorta. Korean Circ J. 2010; 40:475–477.

8. Sasseen BM, Burke JA, Shah R, Costa MA, Zenni M, Gilmore P, et al. Intravascular ultrasound catheter entrapment after coronary artery stenting. Catheter Cardiovasc Interv. 2002; 57:229–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download