Abstract
This article aimed to investigate the incidence rate and possible risk factors for catheter-induced hemorrhage (CIH) after brain parenchymal catheterization. Between January 2011 and March 2013, 381 patients (572 punctures) who underwent brain parenchymal catheterization were retrospectively evaluated. All patients were checked by computerized tomography scan for the detection of hemorrhage within 48 hours after catheter insertion. CIH was defined as any evidence of new hemorrhage on the post-procedural computerized tomography scan. The incidence rate and the possible risk factors were analyzed by surgeon (4 different surgeons performed the procedures), characteristics of the catheter device, and patient background. Of 381 patients, 572 punctures were performed and CIH developed in 122 puncture cases (122/572, 21.3%). The risk factors related to CIH were Glasgow Coma Scale (GCS) score ≤8 (p<0.01) and prothrombin time international normalized ratio (PT INR) ≥1.3 (p=0.038). The amount of hemorrhage was minimal without additional operations. A low GCS score and high PT INR are implicated as potential risk factors for CIH after brain parenchymal catheterization. Careful and delicate operative technique can help to reduce postoperative complications in these patients.
Brain puncture by catheter is thought to be the most commonly used procedure in the neurosurgical field. The procedure is used for external ventricular drainage (EVD), free-hand aspiration (FHA) or stereotactic aspiration for the removal of intracerebral hemorrhage, post-hemorrhagic hydrocephalus, monitoring of intracerebral pressure, ventriculo-peritoneal shunt (V-P shunt), meningitis, and ventriculitis.
In light of this being one of the most frequently practiced operations in the neurosurgical field, it would be logical to further assess the technique, its pitfalls or complications, and its utility. The two most common complications of the brain puncture procedure are related infection and hemorrhage.1,2 Although brain puncture has been performed in many cases, there are sparse reports about catheter-induced hemorrhage (CIH) in contrast with the numerous studies on related infections.1-3 According to reports, the hemorrhagic incidence rate varies from 0% to 41%, representing a significant difference in the result between researchers.4-6 It is a growing trend that medical device manufacturers are developing new catheter devices and are trying to prove the efficacy of these devices regarding the incidence of infection.7 Even though CIH is a more immediate and crucial complication of the procedure, this critical complication has been relatively ignored.
Gardner et al.6 reported that 77 of 188 EVD procedures were associated with imaging evidence of hemorrhage after catheter placement or removal, thus showing a high incidence rate. Furthermore, they showed that 37 of the subjects had a large amount of hemorrhage. Huh et al.8 evaluated the incidence rate and risk factors for the formation of CIH in patients with EVD and reported that age over 60 years, bilateral catheter insertion, and a history of heart disease are the most significant risk factors for CIH. To our knowledge, there are no studies to date evaluating the development of CIH according to interoperator differences and device characteristics such as size or quality of materials. In this study, therefore, we aimed to investigate the incidence of CIH following brain puncture and analyzed three possible risk factors contributing to CIH: the operator, the characteristics of the catheter device, and patient characteristics.
From January 2011 to March 2013, we retrospectively reviewed the medical records of 381 patients (572 cases) who had undergone brain parenchymal catheterization in our neurosurgical clinic. In cases of intracerebral hematoma, FHA or stereotactic aspiration was performed. During the aneurysmal operation, we perform EVD to reduce swollen brain.
All pre-procedural and post-procedural computed tomography (CT) scans were reviewed. We used a method that was introduced by Kothari et al.9 for the estimation of hematoma amount. All patients had the same operating room settings to diminish confounding factors. CIH was defined as any evidence of new hemorrhage along the tract of the catheter or newly developed intracerebral hemorrhage on the post-procedural CT scan obtained within 48 hours of catheter insertion.
We assessed the incidence of CIH according to three categories of risk factors. The first category was the surgeon performing the procedure. We hypothesized that the results of the procedure could differ as a result of surgical skill or the surgeon's experience. The second category was the catheter device itself. We analyzed the incidence rate of CIH according to the catheter manufacturer and size. The third factor were patient factors. We checked the patient's age, history of hypertension, Glasgow Coma Scale (GCS) score, prothrombin time international normalized ratio (PT INR), platelet count, and any medication with antiplatelets or anticoagulants.
The patients who were not checked by consecutive postoperative CT scans and who had a ventricular hemorrhage with parenchymal hemorrhage after operation were excluded from the study. We also excluded patients who had traumatic brain injury, meningitis, and ventriculitis.
The catheters used were made by Korean Sewoon Medical (10.5 Fr, I.D.; 2.1 mm O.D.; 3.5 mm/12 Fr, I.D.; 2.5 mm O.D.; 4.0 mm), Korean Yushin Medical (10.5 Fr, I.D.; 1.9 mm O.D.; 3.5 mm), and Aesculap (7.5 Fr, I.D.; 1.2 mm O.D.; 2.5 mm). We used Aesculap's product in the V-P shunt procedure (67 cases).
We found that 108 of 381 patients (28.3%) who underwent the operation had hematoma (Table 1). We performed 572 brain punctures in 381 patients and found 122 cases (122/572, 21.3%) of CIH that developed in 108 patients. The age range of the patients was from 18 to 84 years old. The age distribution was as follows: ≥65, 327 (57.2%); <65, 245 (42.8%). Among the 572 cases, 227 (39.7%) were males and 345 (60.3%) were females. We found that 50 of 227 males (22%) and 72 of 345 females (20.9%) developed CIH. This difference was not statistically significant (p=0.755).
These can be classified by diseases as follow: 53 cases of aneurysm, 32 cases of intracerebral hematoma (FHA or stereotactic aspiration), 27 cases of EVD, 7 cases of hydrocephalus and 3 cases of FHA+EVD (Table 2).
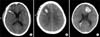
CIH could be classified by the amount of hematoma as follows: 1) 74 cases (74/122, 60.7%) with <1 cc, which mainly occurred along the catheter tract or puncture hemorrhage (Fig. 1A); 2) 42 cases (42/122, 34.4%) with 1 to 5 cc (Fig. 1B); 3) 6 cases (6/122, 4.9%) with more than 5 cc, the largest amount of which was 12.5 cc (Fig. 1C). These hematomas showed no radiological mass effect and no neurologic signs and thus did not require additional treatment.
CIH developed in 65 of 251 (25.9%), 36 of 172 (20.9%), 14 of 93 (15.1%), and 7 of 32 (21.9%) cases according to increasing number of punctures (from one to four punctures, respectively). Among the 122 cases of CIH, 65 cases occurred in patients with one puncture (53.3%), 36 cases in patients with two punctures (29.5%), 14 cases in patients with three punctures (11.5%), and 7 cases in patients with four punctures (5.7%). Thus, we found that the incidence rate of hematoma was not statistically significant with increased number of punctures.
The development of CIH was analyzed by three categories of hemorrhagic risk factors as follows (Table 3).
The surgery was done by four different surgeons with similar surgical experiences of more than 5 years. The rate of CIH incidence was as follows: 1) J.S.L., 60/248 (24.2%); 2) B.C.L., 15/90 (16.7%); 3) R.S.L., 24/119 (20.2%); and 4) K.Y.C., 23/115 (20%). The difference among surgeons was not significant (p=0.314).
We compared and analyzed the development of CIH between Sewoon 10.5 Fr (280 cases) and Yushin 10.5 Fr (153 cases) products, but this turned out to be a statistically insignificant factor in the occurrence rate of hematoma (p=0.277). In addition, we examined the incidence of CIH by catheter size (10.5 Fr vs. 12 Fr), and this was also a statistically insignificant factor (p=0.27).
We analyzed patient age, gender, history of hypertension, GCS score, PT INR, number of platelets in peripheral blood, and antiplatelet or anticoagulant medication (Table 3). Old age over 65 years tended to be related with a high incidence of CIH, but the difference was not statistically significant (p=0.084). CIH developed in 45 of 354 patients (12.7%) with GCS >9 and in 77 of 218 patients (35.3%) with GCS ≤8. This finding showed a high rate of hematoma incidence with poor mentality (p<0.01). In terms of PT INR, CIH was more frequent in patients with PT INR ≥ 1.3, with an incidence rate of 46.2% (6/13) compared with 20.8% (116/559), thus showing a high rate of CIH in patients with prolonged PT INR (p=0.038). There were no significant differences by patient factors such as age, gender, or other patient backgrounds.
The odds that patients with a low GCS score (GCS≤8) developed CIH after the procedure was 3.86 (95% confidence interval [CI]: 2.481-5.988) times that of patients with a higher GCS score (GCS>9; p=0.01; Table 4). The patients who had PT INR ≥1.3 had an approximately 3-fold increased risk of CIH compared with the patients who had PT INR <1.3 (95% CI: 1.079-9.927; p=0.038; Table 4).
In the present study, we investigated the incidence rate and possible risk factors for CIH after brain parenchymal puncture. There was a significant difference in the incidence rate of CIH by GCS and PT INR, which suggests that a low GCS score and high PT INR could be implicated as potential risk factors for CIH after brain parenchymal catheterization. We found no significant differences by surgeon, characteristics of the catheter, puncture time, or other patient background characteristics.
It widely known that complications such as infections or hemorrhage frequently occur after parenchymal brain puncture. Only a few studies have reported the hemorrhagic complication rate after these procedures. In the present study, we found an incidence rate of hemorrhagic complications of 21.3% in 572 punctures. In contrast, Hassler and Zentner5 studied 50 patients retrospectively who underwent procedures with the use of a modified spinal needle and demonstrated a 0% "symptomatic hemorrhage rate." However, most studies showing the rarity of CIH did not include a routine checkup of postoperative CT scans in all patients to determine the real incidence of CIH. Recently, some authors have reported that hemorrhagic complications induced by catheter placement on the brain are more common than previously suspected.6,8 Maniker et al. showed a hemorrhagic complication rate induced by catheter insertion of 33% and they noted that most of the cases rarely caused detectable changes in the neurological examinations.10 Additionally, they documented the need for postoperative CT scans in all patients after the procedure and suggested that even a small hemorrhage without a significant mass effect or clinical symptoms could cause sequels and could be a seizure focus thereafter. Any hemorrhage has the potential to damage associated parenchyma structures or become a nidus for seizure activity.
The results of the present study showed that GCS and PT INR were statistically significant factors in the rate of CIH. In contrast with our result, however, other authors showed that PT INR was not a statistically significant risk factor for the formation of hemorrhage in patients with liver disease.8 The reason for this discrepancy may be the different study populations and study sample sizes. Meanwhile, low GCS was a significant risk factor for the development of CIH. This result may suggest that the possibility of vascular injury can increase during catheter insertion despite high intracranial pressure.
In the present study, age, gender, and puncture time were not significantly related with the development of CIH, but the incidence of CIH tended to increase with increasing age. It has been known that patients with subarachnoid hemorrhage from aneurysmal rupture show a higher risk of CIH, but the exact reason for this phenomenon is unknown.10 In the present study, we also found a high incidence of CIH in patients with subarachnoid hemorrhage from an aneurismal rupture compared with other disease categories.
Moreover, an increasing number of people are taking antiplatelet agents such as aspirin. We suspected that taking antiplatelet medication could be a risk factor for hemorrhage, but we did not find any significant differences in the rate of CIH according to antiplatelet medication use in our study. (In accordance with our study, Wheeler and Bernard11 also reported that the rate of CIH was not significantly different according to antiplatelet medication use, showing CIH rates of 6.9% in patients not on an antiplatelet medication and 21.1% in patients taking an antiplatelet medication).
Other risk factors for CIH could be related to the catheter itself or catheter insertion. In the period of catheter indwelling, micromotion of the catheter in the state of catheterization could affect the incidence of hemorrhage. Also, the procedure of catheter removal could cause injury to the brain that could lead to hemorrhage.6 We secured the catheter to the skin with sutures after insertion to the brain parenchyme, and then the catheter was bent acutely on the burr hole catheter entry point rectangularly. As another catheter factor, characteristics of the catheter might affect the incidence of CIH. The flexibility of the catheter is determined by the manufacturing technique of mixing silicone and a curing agent; furthermore, the roughness of the surface and radius of the tip may be also facors.10 In the present study, however, we could not further compare the incidence of CIH according to detailed characteristics such as flexibility and surface roughness. If the catheter is not flexible, the recoil power of the catheter could act on the brain to cause hemorrhage.
Among the possible risk factors contributing to CIH, we originally assumed that surgical skill could be involved in the formation of CIH. However, we found no differences in the incidence of CIH according to operator factor. In the present study, the surgery was done by four different surgeons with similar surgical experiences of more than 5 years, which may explain this result.
Another important issue related to brain parenchymal puncture with a catheter is the environment of the procedure in which the catheter placement occurs. Gardner et al.6 compared the rates of CIH between operating room and intensive care unit settings. In that study, they found that the intensive care unit setting had a much higher rate of CIH (43.4%) than did the operating room (34.8%). They suggested that proper monitoring with staff members and facilities in the operating room is needed to minimize CIH. These procedures can take place in the operating room, intensive care unit, or bedside. When surgeons conduct the procedure in the operating room, they can concentrate on only the task at hand, with the availability of all necessary equipment, thus making it technically easy. The improved lighting and visualization of the operating field in the operating room may also contribute to reducing hemorrhagic complications.
We excluded patients with traumatic brain injury, ventriculitis, and meningitis from the analysis, because these inflammatory conditions of the brain are often complicated by coagulopathy and can offer significant challenges to the surgeon during the surgical procedure. A recent study reported that traumatic brain injury is related with coagulopathy in 34% of patients with severe traumatic brain injury.11,12 The relevance of the interaction between coagulation and inflammation can easily be detected as disseminated intravascular coagulation and multiple organ failure in response to severe infection, in its most extreme form.
Our study had some limitations. Our study was conducted on the basis of retrospective information on the patients collected at admission; thus, there is a possibility of inaccurate patient history, including medication history of aspirin. We could not analyze the risk factors related to the development of CIH by comparing the groups with CIH development and without CIH development. Thus, we could not represent the exact odds ratio and relative risk of CIH during the procedure. Additional prospective, larger sized studies considering these points may be needed.
In conclusion, low GCS and high PT INR are implicated as potential risk factors for parenchymal hemorrhage after CIH. Careful and delicate operative technique can help to reduce postoperative complications in these patients.
Figures and Tables
FIG. 1
Axial CT scan obtained after catheterization. (A) Minimal hemorrhage is shown along the catheter, demonstrating a small hemorrhage (1-5 cc) (B) and a hemorrhage of more than 5 cc (C).
References
1. Narayan RK, Kishore PR, Becker DP, Ward JD, Enas GG, Greenberg RP, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J Neurosurg. 1982; 56:650–659.
2. Sundbärg G, Nordström CH, Söderström S. Complications due to prolonged ventricular fluid pressure recording. Br J Neurosurg. 1988; 2:485–495.

3. Pfausler B, Spiss H, Beer R, Kampl A, Engelhardt K, Schober M, et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg. 2003; 98:1040–1044.

4. Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918; 68:5–11.

5. Hassler W, Zentner J. Ventricle puncture for external CSF drainage and pressure measurement using a modified puncture needle. Acta Neurochir (Wien). 1988; 94:93–95.

6. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009; 110:1021–1025.

7. Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003; 98:725–730.

8. Huh J, Joo WI, Chough CK, Park HK, Lee KJ, Rha HK. Hemorrhagic complications induced by external ventricular draining catheters. Korean J Cerebrovasc Surg. 2011; 13:256–262.
9. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–1305.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download