Abstract
Little is known about the clinicopathological features of female gastric carcinoma (FGC) patients. We compared the clinicopathologic features and outcomes of FGC patients with curative resection with those of male gastric carcinoma (MGC) patients. We reviewed the hospital records of 940 FGC patients between 1986 and 2005 at Chonnam National University Hospital. Multivariate analysis showed that presence of serosal invasion, lymph node metastasis, and operative type were significant prognostic factors for survival of FGC patients with curative resection. Furthermore, the overall 5-year survival rate of FGC patients with curative resection (53.4%) was higher than that of MGC patients (47.6%, p<0.05). In advanced cases, no significant difference was observed in the overall 5-year survival rate between the FGC and MGC patients (41.6% vs 37.4%, p>0.05). Therefore, serosal invasion, lymph node metastasis, and type of operation were statistically significant parameters associated with survival. Early detection is more important for improving the prognosis of female patients with gastric cancer than for male patients.
It is well known that the incidence of gastric carcinoma is higher for men than for women. Although the incidence of gastric carcinoma varies geographically, men are more commonly affected.1 Several lines of evidence support this lower incidence of gastric carcinoma in women than in men. Some investigators have indicated that there are no differences in survival by sex, whereas others found better survival for women.2-4 Few studies are available concerning the relationship between sex and gastric carcinoma survival. Also, little is known about the clinicopathological features of female gastric carcinoma (FGC) patients. In the present study, we aimed to clarify the magnitude of the gender difference in gastric carcinoma survival and the prognostic factors in women with gastric carcinoma who underwent curative resection.
From 1995 to 2005, 2701 patients with gastric carcinoma were treated curatively by the Department of Surgery, Chonnam National University Hospital. Of these, 940 (34.8%) were FGC patients. There were no exclusion criteria. Information on the patient's age, gender, operative type, tumor size, tumor location, macroscopic appearance, depth of invasion, histologic type, presence of lymph node invasion, and stage at initial diagnosis was obtained from the hospital records. The 6th American Joint Committee on Cancer TNM staging system was used for pathologic staging.5 Histological evaluation was performed according to the Japanese General Rules for Gastric Cancer Study in Surgery and Pathology.6
The survival rates of the patients were calculated by using the Kaplan-Meier method and the relative prognostic importance of the parameters was investigated by using the Cox proportional hazards model. The chi-square test was used to evaluate the statistical significance of differences, and p values less than 0.05 were considered significant.
Table 1 summarizes the clinicopathologic findings of the 940 (34.8%) FGC patients and 1,761 (65.2%) male gastric carcinoma (MGC) patients with curative resection. There was a significant difference in mean age between the FGC patients (55.8 years) and the MGC group (57.4 years) (p<0.001). Subtotal gastrectomy was the procedure most frequently performed (76% of cases) in FGC patients, but there was no significant difference in the operative type between the two groups (Table 1). The mean tumor size was larger in FGC patients (3.7 vs. 3.5 cm), but the difference was not statistically significant (p>0.05). The lower third of the stomach was the most common site of gastric carcinoma in both groups. The most common type of advanced gastric carcinoma in the FGC patients was the ulcerating infiltrative type (386/603, 64.1%). There was no significant difference in macroscopic appearance in early and advanced gastric carcinoma between the two groups. Using the pTNM system, 348 FGC patients were classified as pT1, 154 as pT2, 410 as pT3, and 28 as pT4. According to the grade of anaplasia, 133 tumors were well-differentiated, 184 were moderately differentiated, 441 were poorly differentiated, 48 were mucinous, and 102 were signet ring cell carcinomas. Significantly more FGC patients had a poorly differentiated histology and more MGC patients had a well-differentiated histology (p<0.001). A total of 525 FGC patients were pN0 and 415 had lymph node metastasis. By disease stage, 444, 194, 255, and 47 FGC patients were stage I, II, III, and IV, respectively. The FGC and MGC patients had similar distributions with respect to depth of invasion, nodal involvement, and tumor stage at the initial diagnosis. Of the FGC patients, 638 (67.9%) were classified as either stage I or II at initial diagnosis. Tumor size, depth of invasion, tumor location, operative type, tumor stage at the initial diagnosis, and the presence of lymph node invasion were significant prognostic factors in the univariate analysis (Table 2). The multivariate analysis showed that three factors were independent, statistically significant parameters associated with survival: serosal invasion (risk ratio, 2.485; 95% confidence interval [CI], 1.554-3.792; p<0.001), lymph node metastasis (risk ratio, 2.837; 95% CI, 1.871-4.302; p<0.001), and operative type (risk ratio, 0.554; 95% CI, 0.853-8.915; p<0.01) (Table 3).
The overall 5-year survival rate of the FGC patients was significantly higher than that for the MGC patients (53.4% vs. 47.6%; p=0.010) (Fig. 1). The overall 5-year survival rate of the early FGC patients was significantly higher than that of the MGC patients (94.3% vs. 86.1%; p=0.046) (Fig. 2). The overall 5-year survival rates of advanced FGC and MGC patients with curative resection did not differ significantly (41.63% vs. 37.4%; p=0.077) (Fig. 3).
Although many studies of the prognostic factors in patients with gastric carcinoma have been reported, only a few have examined the prognosis of FGC patients. Therefore, we analyzed the clinicopathologic features of FGC patients undergoing curative resection and their surgical outcomes.
The incidence of gastric carcinoma is known to be higher for men than for women.1,7-9 Chandanos and Lagergren reported that the incidence of gastric carcinoma shows a male dominance, with a male-to-female ratio of about 2:1. They suggested that the male dominance in the incidence of gastric carcinoma can at least be partly explained by a protective effect of estrogen in women.10 In agreement with their result, the male-to-female ratio in the present study was 1.9:1.
There was a difference in histologic differentiation between FGC and MGC patients. Poorly differentiated histology was more common in FGC patients than in MGC patients, as in Yu and Zhao's11 report. However, the use of histologic differentiation as a prognostic factor is another controversial subject, although several studies have examined the prognostic relevance of histologic grade in patients with gastric carcinoma. Furthermore, our study showed that histologic differentiation had no statistical significance as a prognostic factor.
Traditionally, the depth of invasion and the presence or absence of lymph node metastasis are the most important clinicopathologic factors influencing the prognosis of patients with gastric carcinoma.12-14 In the present study, multivariate analysis showed that three factors were independent, statistically significant parameters associated with survival: serosal invasion, lymph node metastasis, and operative type.
Lymph node metastasis is thought to be an important prognostic factor in carcinoma of the stomach. In this study, the lymph node metastasis was an important prognostic factor in FGC patients. Bonezziti et al.2 found that female patients with negative nodes at pT1 or pT2 had better survival. Bando et al.15 also reported that sex should be taken into account as well as clinicopathological variables related to lymph node metastases when determining appropriate therapy for early gastric carcinoma. Schafmayer et al.16 reported that the extent of lymphadenectomy influenced long-time survival in female patients with gastric carcinoma.
In gastric carcinoma, the depth of wall invasion is another important prognostic factor in addition to lymph node metastasis. Moriguchi et al.12 and Adachi et al.14 demonstrated that the depth of wall invasion provides useful prognostic information in patients with gastric carcinoma.
Some studies have suggested that the extent of gastric resection was a significant independent predictor of survival in patients with node-positive gastric carcinoma. Kim et al.17 found that patients who had a distal gastrectomy showed a significantly better long-term prognosis than did patients who underwent total gastrectomy. They interpreted their results in terms of indications for total gastrectomy and the relative risks for more distant and extensive lymph node metastasis.
Several studies have reported a better prognosis for women than for men.2,3,7 Similar to these results, we observed a positive significant effect of female gender on survival outcome in our study. Our results showed that the overall 5-year survival rate of the FGC patients was significantly higher than that for MGC patients (53.4% vs. 47.6%; p=0.010). When FGC patients were divided into those with early and those with advanced carcinoma, the overall 5-year survival rate of the early FGC patients was significantly higher than that of the MGC patients (94.3% vs. 86.1%; p=0.0462). However, the overall 5-year survival rates of advanced FGC and MGC patients with curative resection did not differ statistically (41.63% vs. 37.4%; p=0.0766) in this study. This suggests that early detection is important to achieve better survival rates. With regard to long-term survival, Schafmayer et al.16 reported that no significant difference could be shown between men and women. However, splenectomy had a significant effect on long-term survival between the two groups. When the spleen was preserved, women showed a significantly improved survival rate compared with men with preserved spleens. Therefore, those authors proposed that gender differences should be taken into account when analyzing the long-term data of oncological patients.16 Kim et al.8 proposed that sex hormones such as estrogens contribute to the survival differences between men and women with gastric carcinoma.
In conclusion, serosal invasion, lymph node metastasis, and type of operation were statistically significant parameters associated with survival. Also, we found that the overall 5-year survival rate of FGC patients with curative resection (53.4%) was higher than that of MGC patients. In advanced cases, however, no significant difference was observed in the overall 5-year rate between the FGC and MGC patients. Therefore, early detection is more important for improving the prognosis of female patients with gastric cancer than for male patients.
Figures and Tables
FIG. 1
Survival curves of female and male gastric carcinoma patients with curative resection (male=47.6% vs. female= 53.4%) (p=0.010). F: female, M: male.
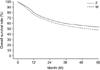
FIG. 2
Survival curves of early female and early male gastric carcinoma (overall 5-year survival rate; male=86.12%, female=94.31%) (p=0.046). F: female, M: male.
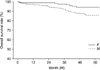
FIG. 3
Survival curves of advanced female and advanced male gastric carcinoma patients with curative resection (male=37.4% vs. female=41.6%) (p=0.077). F: female, M: male.
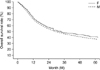
TABLE 1
Clinicopathologic features of female and male gastric carcinoma patients with curative resection

References
1. Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002. 5:213–219.

2. Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S, et al. A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet. 1986. 162:229–234.
3. Korenaga D, Haraguchi M, Okamura T, Baba H, Saito A, Sugimachi K. DNA ploidy and tumor invasion in human gastric cancer. Histopathologic differentiation. Arch Surg. 1989. 124:314–318.

4. Sato N, Ito Y, Ioka A, Tanaka M, Tsukuma H. Gender differences in stomach cancer survival in Osaka, Japan: analyses using relative survival model. Jpn J Clin Oncol. 2009. 39:690–694.

5. American Joint Committee on Cancer. AJCC cancer staging manual. 2002. 6th ed. Springer-Verlag.
6. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998. 1:10–24.
7. Maguire A, Porta M, Sanz-Anquela JM, Ruano I, Malats N, Piñol JL. Sex as a prognostic factor in gastric cancer. Eur J Cancer. 1996. 32A:1303–1309.

8. Kim JH, Boo YJ, Park JM, Park SS, Kim SJ, Kim CS, et al. Incidence and long-term outcome of young patients with gastric carcinoma according to sex: does hormonal status affect prognosis? Arch Surg. 2008. 143:1062–1067.

9. Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007. 56:1671–1677.

10. Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008. 44:2397–2403.

11. Yu J, Zhao Q. The demographic characteristics of histological types of gastric cancer with gender, age, and tumor location. J Gastrointest Cancer. 2009. 40:98–100.

12. Moriguchi S, Odaka T, Hayashi Y, Nose Y, Maehara Y, Korenaga D, et al. Death due to recurrence following curative resection of early gastric cancer depends on age of the patient. Br J Cancer. 1991. 64:555–558.

13. Takeda J, Tanaka T, Koufuji K, Kodama I, Tsuji Y, Kakegawa T. Gastric cancer surgery in patients aged at least 80 years old. Hepatogastroenterology. 1994. 41:516–520.
14. Adachi Y, Mori M, Maehara Y, Kitano S, Sugimachi K. Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg. 1997. 184:373–377.
15. Bando E, Kojima N, Kawamura T, Takahashi S, Fukushima N, Yonemura Y. Prognostic value of age and sex in early gastric cancer. Br J Surg. 2004. 91:1197–1201.

16. Schafmayer C, Jürgens G, Jürgens I, Klomp HJ, Fändrich F, Kahlke V. Long-term survival of curatively operated gastric cancer: influence of the gender and splenectomy. Zentralbl Chir. 2007. 132:515–522.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download