INTRODUCTION
The incidence of esophageal involvement by secondary neoplasms has been reported as 3.2% in autopsy studies of patients with cancer. In most instances, there is direct invasion by the primary tumor from adjacent or contiguous organs.1 Metastatic involvement into the esophagus from a distant primary malignancy is rare but may occur from cancers of the lung, breast, skeletal system, and brain.2
Papillary thyroid cancer accounts for 80% of thyroid cancers and has an optimal prognosis. In fact, the 5-year survival rates (>90-95%) are among the highest of any type of cancer. In several studies, aerodigestive tract invasion by papillary thyroid cancers was reported in approximately 7% to 16% of all cases of thyroid cancer. The sites included all portions of the endolarynx, trachea, esophagus, strap muscles, and recurrent laryngeal nerve.2,3 The patterns of invasion of the tumor are commonly by direct extension of the primary and less commonly by extension of metastatic paratracheal lymph nodes in the tracheoesophageal groove.2 Although infrequent, aerodigestive tract invasion is associated with significant morbidity and in some cases death from uncontrolled locoregional disease. Invasion of these structures produces symptoms of airway insufficiency, dysphagia, and hemoptysis and may increase morbidity by about 5 times or more.4
Recent advancements in endoscopic ultrasonography (EUS) allow the viewing of detailed structural abnormalities and depth of invasion in various gastrointestinal diseases, especially submucosal tumors. These advancements have also helped in the staging of gastrointestinal cancers and in the examination of the pancreato-biliary region.
Here we report a case of papillary thyroid cancer that recurred as an esophageal submucosal tumor in a 75-year-old woman. The tumor was diagnosed as metastatic papillary cancer from the thyroid after surgery. The esophageal submucosal tumor-like recurrence of papillary thyroid cancer and the associated EUS findings have not been reported previously to our knowledge.
CASE REPORT
A 75-year-old woman presented to our hospital with a complaint of an increasing palpable central neck mass for 3 months. She had undergone right hemi-thyroidectomy for papillary thyroid cancer 10 years previously at another hospital. At that time, no distant metastasis was observed. Three years later, a 1-cm sized midline hard neck mass came into being and was diagnosed as metastatic papillary thyroid cancer by gun biopsy. She underwent a total thyroidectomy and central lymph node dissection in Pusan National University Hospital. Histological examination showed no tumor cells in the lymph node specimen. Also, no distant metastasis was found. Since then, she had been taking thyroid hormone replacement. Her thyroid hormone level was checked regularly and had been maintained within the normal range. After surgery, the patient did not want to take radioactive iodine therapy.
At admission, her condition was fair. Her blood pressure, pulse rate, and body temperature were 110/70 mmHg, 68/min, and 36.5℃, respectively. On the physical examination, a 3-cm sized central neck mass was palpable. There were no other abnormal findings on the physical examination. Laboratory findings revealed the following values: white blood cell count, 4,800/mm3; hemoglobin value, 12.4 g/dl; platelet count, 261,000/mm3; ALP, 76 IU/L; AST, 34 IU/L; ALT, 22 IU/L; total bilirubin, 0.54 mg/dl; total protein, 7.0 g/dl; and albumin, 4.3 g/dl. Thyroid function tests revealed the following: TSH, 1.18 µIU/ml (normal range, 0.3-5.0 µIU/ml); T3, 71.3 ng/ml (normal range, 80-170 ng/dl); and free T4, 1.53 ng/dl (normal range, 0.80-2.10 ng/dl). Her thyroglobulin was 398 ng/ml (normal range, 0.0-50.0 ng/ml), and the thyroglobulin level had increased after the second operation.
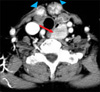
Computed tomography (CT) showed two well-enhanced, ill-defined masses on the anterior aspect of the thyroid bed, which were assumed to be recurrence of thyroid cancer. The CT also showed an approximately 24-mm sized well-defined mass on the left side of the esophagus (Fig. 1). The mass looked like an esophageal submucosal tumor, which differed from typical metastatic papillary cancer. Positron emission tomography-computed tomography (PET-CT) revealed intense hypermetabolic lesions (maximum SUV: 28.0) on both thyroid beds, which were thus thought to be local recurrence of papillary thyroid cancer. The mass on the left side of the upper esophagus also had intense FDG uptake (maximum SUV: 42.4). This finding suggested the possibility of malignancy. No other abnormal glucose uptake was observed.
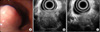
Endoscopy showed a smooth, elevated, cushion sign negative mass covered with intact normal mucosa 2 to 3 cm distal to the upper esophageal sphincter (Fig. 2A). EUS showed a hypoechoic nonhomogeneous 2.7×2.4 cm sized mass in the third (submucosal) and fourth (muscularis propria) layer of the esophagus. The mass was relatively poorly demarcated and included inner hyperechoic spots (Fig. 2B, C). These findings suggested the mass to be a gastrointestinal stromal tumor, but the possibility of it being a metastatic malignant mass could not be ruled out, even though rare.
The patient was transferred to the chest surgery department and underwent a surgical resection. The incision was made through the previous neck incision line. Two masses on the thyroid bed were removed. A discrete mass of about 1.5×1.5 cm in size was exposed on the lateral portion of the left thyroid cartilage. It was an esophageal submucosal mass that focally penetrated the esophageal muscle layer, but within the adventitia. The mass was enucleated and the underlying mucosa was intact. This mass was not connected to the previously removed masses on the thyroid bed. No abnormal lymph nodes were detected.
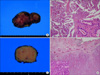
The gross examination of the first specimen removed from the thyroid bed revealed two well-encapsulated masses measuring 6.0×3.5×3.0 cm. The cut surface was papillary with multiple brownish foci of cystic change in appearance, which is the typical morphology of a papillary carcinoma of thyroid origin. Microscopically, the tumor cells exhibited predominantly papillary structures. The nuclei of the tumor cells had typical intranuclear inclusions and groovings. These are typical characteristics of papillary carcinoma cells (Fig. 3A, 3B). The other specimen enucleated from the esophageal wall was a 3.0×2.5×2.0 cm round mass. The cut surface was light brown in color with focal fibrosis and hemorrhage. Microscopically, the tumor was demarcated from the surrounding esophageal muscular layer, but focal muscular invasion was also seen. The tumor cells also showed a papillary pattern in appearance, and the nuclei of the tumor cells also had intranuclear inclusions and groovings (Fig. 3C, D). These findings were consistent with a papillary thyroid carcinoma.
The patient's postoperative course was uneventful and she was discharged 12 days after the surgery. She was referred to the endocrinology department for thyroid hormone replacement and has undergone regular check-ups for recurrence of thyroid papillary cancer. After surgery, her thyroglobulin level was decreased (186 ng/ml). Serial thyroglobulin measurement will be required.
DISCUSSION
Well-differentiated thyroid carcinoma (papillary and follicular subtypes) infrequently invades the upper aerodigestive tract. However, when invasion occurs, it can be a source of significant morbidity as well as mortality for the patient. When cases of anaplastic carcinoma are excluded, the incidence of invasion into the upper aerodigestive tract by well-differentiated thyroid carcinomas is less than 4% and is considered a poor prognostic indicator of survival.4,5
Esophageal involvement by metastatic tumors may occur by direct tumor extension from contiguous organs, through mediastinal lymph nodes containing tumor, and via hematogenous spread.1 Direct extension is the most common route, which is usually seen in tumors of the gastric fundus, hypopharynx, and larynx. Involvement of the esophagus by tumor-containing mediastinal lymph nodes is the second most common route of esophageal invasion by metastatic tumors. Lung and breast carcinomas are the sources of most of these tumors. Hematogenous metastases to the esophagus are very rare and may occur from cancers of the pancreas, testis, eye, tongue, bone, liver, kidney, uterus, skin, synovium, and prostate.1 In this case, papillary thyroid cancer recurred as an esophageal submucosal tumor. Histologic and radiologic findings revealed no lymphangitic metastasis. Although direct invasion was not observed during the third operation, it cannot be excluded. It is possible that during the first and second operations, the esophageal invasion was not removed and, after that, the remaining tumor cells grew. CT is not suitable for evaluation of soft tissue abnormalities; thus, it cannot completely rule out direct invasion. Ohshima et al. reported esophageal invasion of advanced thyroid cancer.6 Five patients with advanced thyroid cancer were evaluated by EUS, and all cases were detected as hypoechoic and irregular masses. Esophageal invasion was confirmed during the operation and histologic analysis. CT and PET-CT are not perfect imaging modalities for the evaluation of soft tissue abnormalities.
Because of the relative resistance of the esophageal mucosa to invasion, gross intraluminal involvement of the esophagus rarely occurs. However, tumors readily penetrate through the esophageal musculature and cause dysphagia secondary to the compressive effects of the tumor mass on the underlying mucosa.1 Corresponding to that, Ohshima et al. reported the destruction of the fourth layer of the esophagus close to the thyroid cancer in a case of esophageal invasion of thyroid cancer detected by EUS.6 At resection, the tumor was dissected from the underlying mucosa without difficulty by developing a submucosal plane. CT and magnetic resonance imaging are useful methods for evaluating the thyroid and adjacent tissue. However, EUS is more suitable than other modalities for detecting the infiltration or metastasis of thyroid cancer.6
Recent advances in EUS have made detailed evaluation of submucosal tumors of the esophagus possible. In our patient, the mass of the esophagus appeared as a submucosal mass originating from the esophageal wall, but showed several differences in the EUS findings from the typical appearance of esophageal submucosal tumors. Gastrointestinal stromal tumors are typically hypoechoic lesions with well-defined margins; they rarely have irregular margins and ulcerations. Most gastrointestinal stromal tumors originate from the muscularis propria. Infrequently, gastrointestinal stromal tumors appear inhomogeneous due to liquefaction necrosis, connective tissue, or cystic and hyaline degeneration. The mass in our case was located in the submucosal and muscularis propria layer, its margin was relatively poorly demarcated, and it appeared more hyperechoic than do ordinary gastrointestinal stromal tumors. These findings could help to differentiate metastatic cancers from gastrointestinal stromal tumors.
There are few cases of esophageal metastasis of thyroid cancer. One case of esophageal metastasis of thyroid cancer that presented with hematemesis was reported.7 There was also a case of occult papillary thyroid cancer that invaded the trachea and esophagus and was diagnosed by brohchoscopy.8 Furthermore, a case of esophageal metastasis of papillary thyroid cancer that presented with progressive dysphagia was also reported.9
In our case, the recurrence of thyroid cancer to the esophagus happened seven years after the total thyroidectomy and it did not induce any symptoms. The patient presented with a palpable neck mass, but that symptom was caused by the masses that recurred on the thyroid bed. To our knowledge, esophageal submucosal tumor-like recurrence of papillary thyroid cancer and the associated EUS findings have not been reported previously.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download